Last update 16 May 2024
IT-141
Last update 16 May 2024
Overview
Basic Info
Drug Type Small molecule drug |
Synonyms SN-38-nanoparticles-Intezyne-Technologies + [3] |
Target |
Mechanism CES2 modulators(carboxylesterase 2 modulators), TOP1 inhibitors(DNA topoisomerase I inhibitors) |
Therapeutic Areas |
Active Indication |
Inactive Indication |
Originator Organization |
Active Organization |
Inactive Organization |
Drug Highest PhasePhase 1 |
First Approval Date- |
Regulation- |
Structure
Molecular FormulaC22H20N2O5 |
InChIKeyFJHBVJOVLFPMQE-QFIPXVFZSA-N |
CAS Registry86639-52-3 |
Related
5
Clinical Trials associated with IT-141A Phase I, Open-label, Dose-finding Study to Assess the Safety, Tolerability, Pharmacokinetics and Preliminary Efficacy of TRX-920 Oral Gel (10 mg and 30 mg) in Patients With Advanced Solid Tumors
The study drug TRX-920 Oral Gel contains SN38, an active metabolite of Irinotecan (CPT-11), which is a widely prescribed anti-cancer drug that has been approved in many countries for the treatment of colorectal and pancreatic cancer. TRX-920 is the oral gel formulation that directly contains SN38 instead of Irinotecan. A series of biology and animal studies have demonstrated that the TRX-920 Oral Gel could inhibit tumor growth with fewer side effects compared to Irinotecan.
Start Date31 Oct 2023 |
Sponsor / Collaborator |
An Open-Label, Multicenter, Non-Randomized, Dose-Escalation, phase 1, Study to Evaluate Safety and Efficacy of Intravesical SN-38 Lipid Suspension in Patients with Non-Muscle Invasive Bladder Cancer. - NA
Start Date30 May 2022 |
Sponsor / Collaborator |
A Phase 1 With Expansion Cohort, Open-Label, Dose Escalation Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Efficacy of Intravenously Infused IT-141 in Subjects With Recurrent or Refractory Solid Tumors
IT141 is a novel nanoparticle formulation of SN-38, the active metabolite of irinotecan, and is intended to deliver more drug to the tumor with reduced toxicity on normal tissues. The study is designed to determine the maximum tolerated dose (MTD) of IT-141, and to investigate pharmacokinetic (PK) parameters and possible pharmacodynamics (PD) relationships. Patients will also be monitored for any response to therapy.
Start Date23 Mar 2017 |
Sponsor / Collaborator |
100 Clinical Results associated with IT-141
Login to view more data
100 Translational Medicine associated with IT-141
Login to view more data
100 Patents (Medical) associated with IT-141
Login to view more data
1,523
Literatures (Medical) associated with IT-14101 May 2024·Bioorganic Chemistry
Magnolol derivatives as specific and noncytotoxic inhibitors of breast cancer resistance protein (BCRP/ABCG2)
Article
Author: Henrique Kita, Diogo ; Ambudkar, Suresh V ; da Silva Zanzarini, Isadora ; Rotuno Moure, Vivian ; Cardullo, Nunzio ; Gomes de Moraes Rego, Fabiane ; Valdameri, Glaucio ; de Paula Dutra, Julia ; Karoline Dos Santos, Kelly ; Muccilli, Vera ; Picheth, Geraldo ; Augusto Pastore, Matteo ; Scheiffer, Gustavo ; Pulvirenti, Luana ; Tringali, Corrado
01 May 2024·Analytica Chimica Acta
Quantifying payloads of antibody‒drug conjugates using a postcolumn infused-internal standard strategy with LC‒MS
Article
Author: Lu, Yen-Shen ; Chen, I-Chun ; Lin, Ching-Hung ; Kuo, Ching-Hua ; Liao, Hsiao-Wei ; Chang, Wen-Chi ; Cheng, Chih-Ning
15 Apr 2024·International Journal of Cancer
Article
Author: Zhu, Ji ; Wang, Shuo-Wen ; Fan, Jin ; Jiang, Pei-Cheng ; Li, Chao
51
News (Medical) associated with IT-14110 May 2024
SEOUL, South Korea, May 9, 2024 /PRNewswire/ -- SN Bioscience Co. Ltd. (CEO Park Young-hwan) announced on May 7 that the FDA has granted Fast Track Designation for small cell lung cancer (SCLC) for SNB-101 (API: SN-38), a new drug for polymer nanoparticle anticancer under clinical trial. SNB-101 was designated as an orphan drug for small cell lung cancer and pancreatic cancer in July of last year and February of this year, respectively. By receiving fast-track designation this time, it is evaluated that it has laid the groundwork that can be commercialized immediately after completion of phase 2 clinical trials.
Continue Reading
SN Bioscience Receives FDA Fast Track Designation for Small Cell Lung Cancer
Despite a long period of research and development, SCLC still remains a field with high medical unmet needs. Currently, the first-line standard treatment is a combination therapy of cisplatin and etoposide, a classic cytotoxic anticancer drug, and 'clinical trials' are included as second-line treatments in the NCCN guidelines.
Fast track is a process designed to facilitate the development and expedite the review of drugs to treat serious conditions and fill an unmet medical need. The Fast Track designation facilitates the interactions with the FDA and allows a rolling review for the submission package so it can be reviewed in each section, rather than waiting until every section of the NDA is completed. Additionally, it may be possible to apply for accelerated approval after the completion of phase 2 clinical trials and priority review immediately after the completion of phase 3 clinical trials when qualified.
SNB-101 is the world's first nanoparticle anticancer drug that has been developed extremely insoluble SN-38 into polymer nanoparticles. The nano micelle technology, a core platform technology of SN Bioscience, has been applied. Preclinical and phase 1 clinical results showed that it significantly reduced digestive system adverse events (nausea, vomiting, diarrhea, etc.) compared to existing anticancer drugs, and especially showed excellent efficacy in patients related to lung cancer through lung targeting. The phase 1 clinical trial has been completed, IND for phase 2 has been approved in Korea, and global clinical trials are scheduled to begin after IND approval for phase 2 in the US and Europe in the second half of this year. Following small cell lung cancer and pancreatic cancer, attempts are being made to expand its indications to other solid cancers such as colon cancer, gastric cancer, and biliary tract cancer, and will be verified through phase 2 clinical trials.
About SN BioScience Inc.
SN BioScience is a biotech company established in May 2017. It is a drug delivery system R&D company specialized in anti-cancer drugs and is located in the 2nd Pangyo Techno Valley in Seongnam-si, Gyeonggi-do, Korea. SN BioScience was founded by pharmaceutical R&D experts, world-class bio-polymer research professors, and clinical professors. From the beginning of its establishment, it has focused on "commercialization" and has been developing nanoliposomes and nanoparticle drug carriers based on pharmacometrics and pharmacokinetics.
[SNB-101]
The development name, SNB-101, is the world's first anticancer drug developed from SN-38 as nanoparticles. SN-38 is an active metabolite of Irinotecan which is gaining attention for its use in drug-antibody conjugates (ADCs) such as Enhertu® and Trodelvy®. Tolerability and safety have been dramatically improved compared to existing products, and it is expected to be effective in lung cancer, pancreatic cancer, and gastric cancer, were not the indications before. Scale-up production, the biggest barrier that prevented existing nano-cancer drugs from entering the clinical stage, was successful, and clinical trial products are produced at a facility dedicated to anticancer drugs with EU GMP.
SOURCE SN BioScience Inc

Fast TrackPhase 1Phase 2Clinical ResultOrphan Drug
09 May 2024
Presentation of interim TAMARACK Phase 2 study data: updated safety and preliminary efficacy of vobra duo in mCRPC patientsConference call scheduled for today at 4:30 p.m. ET ROCKVILLE, Md., May 09, 2024 (GLOBE NEWSWIRE) -- MacroGenics, Inc. (NASDAQ: MGNX), a biopharmaceutical company focused on discovering, developing, manufacturing and commercializing innovative antibody-based therapeutics for the treatment of cancer, today provided an update on its recent corporate progress and reported financial results for the quarter ended March 31, 2024. “We are very encouraged by the interim updated safety and preliminary efficacy data from the TAMARACK study of vobra duo in metastatic castration-resistant prostate cancer," said Scott Koenig, M.D., Ph.D., President and CEO of MacroGenics. “We believe this interim data set helps validate our previously stated hypothesis that improved tolerability coupled with compelling biological activity could be achieved through dose reductions and a longer dosing interval. We believe vobra duo’s biological activity shown to date aligns well with the parameters we outlined at the outset of the study. Based on our evaluation of the interim data to date, we have initiated planning activities for a potential Phase 3 study that could commence next year. We anticipate sharing final safety, efficacy and durability data, including radiographic progression-free survival data, which is the primary endpoint of the study, in the second half of 2024. Furthermore, having preliminarily identified suitable vobra duo doses in mCRPC in the TAMARACK study, we have greater confidence in the molecule’s potential to help patients with a broad range of B7-H3-expressing cancers.” “The interim safety and anti-tumor activity observed to date in the TAMARACK study look very promising for patients with metastatic castration-resistant prostate cancer,” said Johann DeBono, Regius Professor of Cancer Research and Professor in Experimental Cancer Medicine at The Institute of Cancer Research, London and The Royal Marsden NHS Foundation Trust. “With the limited treatment options currently available to these patients, this novel ADC molecule could potentially become the first therapy targeting B7-H3 in patients with prostate cancer and would represent an important new treatment for this population.” Updates on Proprietary Investigational Programs Recent progress and anticipated events related to MacroGenics’ investigational product candidates are highlighted below. B7-H3-Directed Therapies •Vobramitamab duocarmazine (vobra duo) is an antibody-drug conjugate (ADC) that targets B7-H3, an antigen with broad expression across multiple solid tumors and a member of the B7 family of molecules involved in immune regulation. •MacroGenics completed enrollment of the TAMARACK Phase 2 study of vobra duo in November 2023. TAMARACK is being conducted in patients with metastatic castration-resistant prostate cancer (mCRPC) who were previously treated with one prior androgen receptor axis-targeted therapy (ARAT). Participants may have received up to one prior taxane-containing regimen, but no other chemotherapy agents. The TAMARACK study is designed to evaluate vobra duo at two different doses: 2.0 mg/kg or 2.7 mg/kg every four weeks (q4W). •A new presentation of TAMARACK interim data, consisting of updated safety and preliminary efficacy data, all based on a data cut-off date of April 12, 2024, is available under "Events & Presentations" in the Investor Relations section of MacroGenics’ website or directly via this link. Below is a high-level summary of this interim data, which is subject to further updates: Vobra Duo2.0 mg/kg q4WVobra Duo2.7 mg/kg q4WPatients Enrolledn=91n=90PSA Reduction Summary: PSA Evaluable Patientsn=82n=71Any PSA Reduction ≥50%41 (50.0%)36 (50.7%)Confirmed PSA Reduction ≥50%36 (43.9%)26 (36.6%)Tumor Response Summary: RECIST Evaluable Patients with Measurable Disease at Baselinen=45n=32Disease Control Rate (CR+PR+SD)41 (91.1%)28 (87.5%)Overall Response Rate (CR+PR, confirmed only)8 (17.8%)8 (25.0%)Overall Response Rate (CR+PR, including unconfirmed)11 (24.4%)14 (43.8%)Safety Summary: Safety Populationn=90n=86Treatment-Emergent Adverse Events All Grade89 (98.9%)86 (100.0%)Treatment- Emergent Adverse Events Grade ≥349 (54.4%)44 (51.2%)TEAE Leading to Study Drug Discontinuation10 (11.1%)13 (15.1%)TEAE Leading to Study Drug Dose Reduction39 (43.3%)44 (51.2%)TEAE Leading to Study Drug Dose Interruption38 (42.2%)48 (55.8%)Five Most Common TEAE All GradeAsthenia (46.7%)Nausea (35.6%)Oedema peripheral (32.2%) Decreased appetite (28.9%) Fatigue (25.6%)Asthenia (58.1%)Decreased appetite (37.2%) Oedema peripheral (36.0%) Nausea (30.2%)Pleural effusion (29.1%)Pleural EffusionsGrade 1=8.9%Grade 2=8.9%No Grade ≥ 3 eventGrade 1=14.0%Grade 2=14.0%Grade 3=1.2%Palmar-plantar Erythrodysaesthesia SyndromeGrade 1=11.1%Grade 2=4.4%No Grade ≥ 3 eventGrade 1=12.8%Grade 2=9.3%Grade 3=1.2% • The median number of cycles of vobra duo administered was five (range of 1-10).
• A total of five events with fatal outcome occurred as follows: one Grade 5 event in the 2.0 mg/kg dosing cohort: acute myocardial infarction (considered unrelated to study drug by the investigator); three Grade 5 events in the 2.7 mg/kg dosing cohort: one cardiac arrest (considered unrelated to study drug by the investigator) and two events of pneumonitis. In addition, a patient in the 2.7 mg/kg dosing cohort had a Grade 3 pleural effusion that is recorded as having a fatal outcome. The latter three deaths are being investigated, as follow-up is incomplete on this ongoing trial.
• Additional data is provided in the presentation on the Company’s website and as filed with the Securities and Exchange Commission.
• Based on a current evaluation of this interim data, the Company is undertaking the initial steps necessary to prepare for the potential initiation of a Phase 3 study in mCRPC in 2025. The final decision to pursue such a Phase 3 study will be based on an analysis of the final data set, including rPFS, when available.
•The Company intends to share final safety, efficacy, and durability data, including the primary endpoint of radiographic progression-free survival, from the TAMARACK trial in the second half of 2024.
•MacroGenics plans to expand the TAMARACK study of vobra duo by enrolling patients with non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), melanoma, squamous cell carcinoma of the head and neck (SCCHN) and anal cancer. The Company expects to initiate dosing in these additional cohorts in mid-2024.
•MacroGenics continues to enroll a Phase 1/2 dose escalation study of vobra duo in combination with lorigerlimab in patients with various advanced solid tumors. The Company anticipates commencing a dose expansion study of this combination in mCRPC and at least one additional indication in 2024. •MGC026 is a clinical B7-H3-targeting ADC that is site-specifically conjugated to exatecan, a topoisomerase I inhibitor payload developed by Synaffix (a Lonza company). With distinct mechanisms of action, vobra duo and MGC026 may address different cancers, tumor stages, or be used in combination with alternate agents — or potentially with one another — to enhance their clinical utility. A Phase 1 dose escalation study of MGC026 in patients with advanced solid tumors is ongoing.MGC026 preclinical data was presented recently at the American Association for Cancer Research (AACR) Annual Meeting. In preclinical studies, MGC026 was shown to have greater potency than B7-H3-directed antibodies conjugated to deruxtecan, or DXd, a topoisomerase-based payload utilized in other ADCs. In addition, the MGC026 payload has been shown to be less susceptible to multi-drug resistance (MDR) mechanisms than DXd and SN-38. •Enoblituzumab is an Fc-optimized monoclonal antibody that targets B7-H3. The HEAT study, an investigator-sponsored, randomized Phase 2 clinical trial being conducted by MacroGenics’ academic collaborators, is ongoing. This study is being conducted to evaluate the activity of neoadjuvant enoblituzumab given prior to radical prostatectomy in up to 219 men with high-risk localized prostate cancer. Lorigerlimab •Lorigerlimab is a bispecific, tetravalent PD-1 × CTLA-4 DART® molecule. In addition to the ongoing study of lorigerlimab in combination with vobra duo mentioned above, MacroGenics is enrolling LORIKEET, a randomized Phase 2 study of lorigerlimab in combination with docetaxel vs. docetaxel alone in second-line, chemotherapy-naïve mCRPC patients. A total of 150 patients are planned to be treated in the 2:1 randomized study. The current trial design includes a primary study endpoint of radiographic progression-free survival (rPFS). The Company anticipates completing enrollment of the study in 2024 and providing a clinical update in the first half of 2025. Emerging ADC Pipeline •MGC028 is a preclinical ADC incorporating an ADAM9-targeting antibody and represents the second MacroGenics ADC molecule that incorporates Synaffix’s novel site-specific linker and topoisomerase I inhibitor-based cytotoxic payload. ADAM9 (a disintegrin and metalloprotease domain 9) is a member of the ADAM family of multifunctional type 1 transmembrane proteins that play a role in tumorigenesis and cancer progression and is overexpressed in multiple cancers, making it an attractive target for cancer treatment. The Company currently anticipates submitting an investigational new drug (IND) application for MGC028 by the end of 2024.MGC028 preclinical data was presented recently at the AACR Annual Meeting. In preclinical studies, MGC028 demonstrated specific antitumor activity in in vivo models representing gastric, lung, pancreatic, colorectal cancer, SCCHN and cholangiocarcinoma. In addition, in a non-human primate study, MGC028 was well tolerated at high dose levels, with mild, reversible side effects and no ocular toxicity, which is often a concern with tubulin-inhibitor-based ADCs. These promising preclinical results support the continued investigation of MGC028 as a therapeutic option for treating ADAM9-expressing solid cancers. Partnered Program •MGD024 is a next-generation, humanized CD123 × CD3 DART molecule designed to minimize cytokine-release syndrome, while maintaining anti-tumor cytolytic activity, and permitting intermittent dosing. MacroGenics continues to enroll patients in a Phase 1 dose-escalation study of MGD024 in patients with CD123-positive neoplasms, including acute myeloid leukemia and myelodysplastic syndromes. Under an October 2022 exclusive option and collaboration agreement, Gilead Sciences, Inc. has the option to license MGD024 at predefined decision points during the Phase 1 study. First Quarter 2024 Financial Results •Cash Position: Cash, cash equivalents and marketable securities balance as of March 31, 2024, was $184.2 million, compared to $229.8 million as of December 31, 2023. •Revenue: Total revenue was $9.1 million for the quarter ended March 31, 2024, compared to total revenue of $24.5 million for the quarter ended March 31, 2023. The decrease was primarily due to a decrease in revenue from collaborative and other agreements, including a $15.0 million milestone received from Incyte in the quarter ended March 31, 2023. •R&D Expenses: Research and development expenses were $46.0 million for the quarter ended March 31, 2024, compared to $45.9 million for the quarter ended March 31, 2023. •SG&A Expenses: Selling, general and administrative expenses were $14.7 million for the quarter ended March 31, 2024, compared to $13.5 million for the quarter ended March 31, 2023. The increase was primarily related to increased stock-based compensation expense and other professional fees. •Net Loss: Net loss was $52.2 million for the quarter ended March 31, 2024, compared to net loss of $38.0 million for the quarter ended March 31, 2023. •Shares Outstanding: Shares of common stock outstanding as of March 31, 2024 were 62,560,502. •Cash Runway Guidance: MacroGenics anticipates that its cash, cash equivalents and marketable securities balance of $184.2 million as of March 31, 2024, in addition to projected and anticipated future payments from partners and product revenues should extend its cash runway into 2026. The Company’s expected funding requirements reflect anticipated expenditures related to the Phase 2 TAMARACK clinical trial, the Phase 2 LORIKEET study as well as MacroGenics’ other ongoing clinical and preclinical studies. Conference Call Information To participate via telephone, please register in advance at this link. Upon registration, all telephone participants will receive a confirmation email detailing how to join the conference call, including the dial-in number along with a unique passcode and registrant ID that can be used to access the call. The listen-only webcast of the conference call can be accessed under "Events & Presentations" in the Investor Relations section of MacroGenics’ website at http://ir.macrogenics.com/events.cfm. A recorded replay of the webcast will be available shortly after the conclusion of the call and archived on MacroGenics’ website for 30 days following the call. MACROGENICS, INC.SELECTED CONSOLIDATED BALANCE SHEET DATA(Amounts in thousands) March 31, 2024 December 31, 2023 (unaudited) Cash, cash equivalents and marketable securities$184,237 $229,805Total assets 248,285 298,418Deferred revenue 79,019 80,894Total stockholders' equity 106,154 152,613 MACROGENICS, INC.CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS(Unaudited)(Amounts in thousands, except share and per share data) Three Months Ended March 31, 2024 2023 Revenues: Collaborative and other agreements$1,449 $16,686 Product sales, net 4,861 3,490 Contract manufacturing 2,276 3,615 Royalty revenue 160 422 Government agreements 358 283 Total revenues 9,104 24,496 Costs and expenses: Cost of product sales 270 113 Cost of manufacturing services 1,846 3,410 Research and development 46,029 45,872 Selling, general and administrative 14,709 13,527 Total costs and expenses 62,854 62,922 Loss from operations (53,750) (38,426)Interest and other income 2,693 1,073 Interest and other expense (1,133) (656)Net loss (52,190) (38,009)Other comprehensive income (loss): Unrealized gain (loss) on investments (29) 13 Comprehensive loss$(52,219) $(37,996) Basic and diluted net loss per common share$(0.84) $(0.61)Basic and diluted weighted average common shares outstanding 62,290,538 61,809,817
About MacroGenics, Inc. MacroGenics (the Company) is a biopharmaceutical company focused on discovering, developing, manufacturing and commercializing innovative monoclonal antibody-based therapeutics for the treatment of cancer. The Company generates its pipeline of product candidates primarily from its proprietary suite of next-generation antibody-based technology platforms, which have applicability across broad therapeutic domains. The combination of MacroGenics' technology platforms and protein engineering expertise has allowed the Company to generate promising product candidates and enter into several strategic collaborations with global pharmaceutical and biotechnology companies. For more information, please see the Company's website at www.macrogenics.com. MacroGenics, the MacroGenics logo, MARGENZA and DART are trademarks or registered trademarks of MacroGenics, Inc. Cautionary Note on Forward-Looking Statements Any statements in this press release about future expectations, plans and prospects for MacroGenics (“Company”), including statements about the Company’s strategy, future operations, clinical development of the Company’s therapeutic candidates, including initiation and enrollment in clinical trials, expected timing of results from clinical trials, discussions with regulatory agencies, commercial prospects of or product revenues from MARGENZA and the Company’s product candidates, if approved, manufacturing services revenue, milestone or opt-in payments from the Company’s collaborators, the Company’s anticipated milestones and future expectations and plans and prospects for the Company, as well as future global net sales of TZIELD and the Company’s ability to achieve the milestone payments set forth under the terms of the agreement with DRI (or its successors or assigns with respect to such agreement), and other statements containing the words “subject to”, "believe", “anticipate”, “plan”, “expect”, “intend”, “estimate”, “potential,” “project”, “may”, “will”, “should”, “would”, “could”, “can”, the negatives thereof, variations thereon and similar expressions, or by discussions of strategy constitute forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including: risks that TZIELD, vobramitamab duocarmazine, lorigerlimab, ZYNYZ, MARGENZA or any other product candidate’s revenue, expenses and costs may not be as expected, risks relating to TZIELD, vobramitamab duocarmazine, lorigerlimab, ZYNYZ, MARGENZA or any other product candidate’s market acceptance, competition, reimbursement and regulatory actions; future data updates, especially with respect to vobramitamab duocarmazine; our ability to provide manufacturing services to our customers; the uncertainties inherent in the initiation and enrollment of future clinical trials; the availability of financing to fund the internal development of our product candidates; expectations of expanding ongoing clinical trials; availability and timing of data from ongoing clinical trials; expectations for the timing and steps required in the regulatory review process; expectations for regulatory approvals; expectations of future milestone payments; the impact of competitive products; our ability to enter into agreements with strategic partners and other matters that could affect the availability or commercial potential of the Company's product candidates; business, economic or political disruptions due to catastrophes or other events, including natural disasters, terrorist attacks, civil unrest and actual or threatened armed conflict, or public health crises such as the novel coronavirus (referred to as COVID-19 pandemic); and other risks described in the Company's filings with the Securities and Exchange Commission. In addition, the forward-looking statements included in this press release represent the Company's views only as of the date hereof. The Company anticipates that subsequent events and developments will cause the Company's views to change. However, while the Company may elect to update these forward-looking statements at some point in the future, the Company specifically disclaims any obligation to do so, except as may be required by law. These forward-looking statements should not be relied upon as representing the Company's views as of any date subsequent to the date hereof. CONTACTS: Jim Karrels, Senior Vice President, CFO 1-301-251-5172 info@macrogenics.com
Phase 2Phase 1Clinical ResultFinancial StatementAACR
07 May 2024
Non-Small Cell Lung Cancer Development Program Data to be Presented, Including the Phase 3 EVOKE-01 Study
Updated Results from Phase 2 EDGE-Gastric Study of Fc-Silent Anti-TIGIT Domvanalimab Plus Anti-PD-1 Zimberelimab, to be Presented
Pilot Study Results of Yescarta® in Relapsed/Refractory Primary and Secondary Central Nervous System Lymphomas, an Area of Unmet Clinical Need, to be Presented Orally
FOSTER CITY, Calif. & SANTA MONICA, Calif.--(BUSINESS WIRE)-- Gilead Sciences, Inc. (Nasdaq: GILD) and Kite, a Gilead Company, will present 18 abstracts during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting. These data showcase the ongoing commitment to developing differentiated approaches to transform how cancer is treated and span solid tumors and blood cancers, including lung, breast, gastrointestinal, colorectal, leukemia and lymphoma.
This press release features multimedia. View the full release here:
New Data Provide Detail on Trodelvy in Non-Small Cell Lung Cancer (NSCLC)
Gilead is presenting primary results from the global Phase 3 EVOKE-01 study of Trodelvy® (sacituzumab govitecan-hziy) versus docetaxel in patients with advanced or metastatic NSCLC that has progressed on or after platinum-based chemotherapy and checkpoint inhibitor therapy. In addition, Gilead will present longer-term results from Cohort A of the Phase 2 EVOKE-02 study of Trodelvy in combination with KEYTRUDA® (pembrolizumab) in first-line advanced or metastatic squamous/non-squamous PD-L1-high NSCLC.
Gilead Highlights Progress in Gastrointestinal Pipeline
An updated analysis from the Phase 2 EDGE-Gastric study will be featured as a rapid oral presentation in partnership with Arcus Biosciences. These longer-term efficacy and safety results for domvanalimab, an Fc-silent anti-TIGIT antibody, plus the anti-PD-1 antibody zimberelimab and chemotherapy, as a potential first-line treatment for upper gastrointestinal cancers follow the encouraging ORR and six-month PFS rate results from the preliminary analysis presented during the November 2023 virtual ASCO Plenary Series.
Additionally, an oral presentation with our partner Arcus Biosciences will feature data from Cohort B of ARC-9, a Phase 1b/2 study evaluating the safety and efficacy of etrumadenant, a dual A2a/b adenosine receptor antagonist, plus zimberelimab, and FOLFOX/bevacizumab in third-line metastatic colorectal cancer (mCRC).
Kite Showcases New Data on CAR T-cell Therapies
A pilot collaborative study evaluating Yescarta® (axicabtagene ciloleucel) for the treatment of patients with relapsed/refractory (R/R) primary and secondary central nervous system lymphoma (PCNSL and SCNSL) will be presented orally. These updated data will highlight the efficacy and safety of Yescarta in this patient population with important unmet need.
Additionally, updated overall survival outcomes for Tecartus® (brexucabtagene autoleucel) from the pivotal Phase 1/2 ZUMA-3 trial, now with more than four years of follow-up, will be presented. The ZUMA-3 trial is the longest follow-up in an adult-only study of a CAR T-cell therapy for R/R B-cell lymphoblastic leukemia (B-ALL). These data continue to support the long-term survival and durability of Tecartus.
Summary of Presentations
Accepted abstracts at the 2024 ASCO Annual Meeting include:
Tumor Types
Abstract Title
B-cell Acute Lymphoblastic Leukemia
Abstract #6531
June 3, 2024
9:00 AM – 12:00 PM CDT
(Poster)
Long-term Survival Outcomes of Patients (pts) with Relapsed or Refractory B-cell Acute Lymphoblastic Leukemia (R/R B-ALL) Treated with Brexucabtagene Autoleucel (brexu-cel) in ZUMA-3
Biliary Tract Cancer
Abstract #TPS4195
June 1, 2024
1:30 – 4:30 PM CDT (TiP)
Phase 2 Study of Gemcitabine, Cisplatin, Quemliclustat (AB680) and Zimberelimab (AB122) During First-Line Treatment of Advanced Biliary Tract Cancers (BTC) – Big Ten Cancer Research Consortium Study BTCRC-GI22-564
Breast Cancer
Abstract #LBA1004
June 1, 2024
3:00-6:00 PM CDT (Oral Presentation)
SACI-IO HR+: A Randomized Phase II Trial of Sacituzumab Govitecan with or without Pembrolizumab in Patients with Metastatic Hormone Receptor-positive/HER2-negative Breast Cancer*
Abstract #1101
June 2, 2024
9:00 AM – 12:00 PM CDT (Poster)
Prevention of Sacituzumab Govitecan (SG)-related Neutropenia and Diarrhea in Patients (pts) with Triple-negative or HR+/HER2- Advanced Breast Cancer (ABC) (PRIMED): a Phase 2 Trial**
Abstract #1075
June 2, 2024
9:00 AM – 12:00 PM CDT (Poster)
Genomic Alterations in DNA Damage Response (DDR) Genes in HR+/HER2- Metastatic Breast Cancer (mBC) and Impact on Clinical Efficacy with Sacituzumab Govitecan (SG): Biomarker Results from TROPiCS-02 Study
Abstract #1102
June 2, 2024
9:00 AM – 12 PM CDT
(Poster)
Sequential Combination of Sacituzumab Govitecan and PARP Inhibitor Talazoparib in Metastatic Triple Negative Breast Cancer (mTNBC): Results from the Phase II Study
Abstract #TPS1140
June 2, 2024
9:00 AM – 12 PM CDT
(TiP)
Trial in Progress: Phase I/II Study of Stereotactic Radiation and Sacituzumab Govitecan with Zimberelimab in the Management of Metastatic Triple Negative Breast Cancer with Brain Metastases
Central Nervous System Lymphoma
Abstract #2006
June 3, 2024
8:00 – 11:00 AM CDT
(Oral Presentation)
A Pilot Study of Axicabtagene Ciloleucel (axi-cel) for the Treatment of Relapsed/Refractory Primary and Secondary Central Nervous System Lymphoma (PCNSL and SCNSL)*
Colorectal Cancer
Abstract #3508
June 2, 2024
8:00 – 11:00 AM CDT (Oral Presentation)
Randomized Phase 1b/2 Study to Evaluate Etrumadenant Based Treatment Combinations in Previously Treated Metastatic Colorectal Cancer (mCRC)
Gastroesophageal Cancers
Abstract #433248
June 1, 2024
12:30 – 1:30 PM CDT (Oral Presentation)
EDGE-Gastric Arm A1: Phase 2 Study of Domvanalimab, Zimberelimab, and FOLFOX in First-Line Advanced Gastroesophageal Cancer
Lung Cancer
Abstract #LBA8500
May 31, 2024
2:45 – 5:45 PM CDT (Oral Presentation)
Sacituzumab Govitecan (SG) vs Docetaxel (doc) in Patients (pts) with Metastatic Non-small Cell Lung Cancer (mNSCLC) Previously Treated with Platinum (PT)-based Chemotherapy (chemo) and PD(L)-1Inhibitors (IO): Primary Results from the Phase 3 EVOKE-01 Study
Abstract #8592
June 3, 2024
1:30 – 4:30 PM CDT
(Poster)
Sacituzumab Govitecan (SG) + Pembrolizumab (pembro) in First-line (1L) Metastatic Non-small Cell Lung Cancer (mNSCLC): Results from Longer Follow-up of Cohort A of EVOKE-02
Abstract #TPS8121
June 3, 2024
1:30 – 4:30 PM CDT (TiP)
VELOCITY-Lung Substudy-03: a Phase 2 Study Evaluating Safety and Efficacy of Domvanalimab (Dom) + Zimberelimab (Zim) or Zim Alone in Combination with Chemotherapy (Chemo) in the Neoadjuvant Phase and Dom+Zim or Zim Alone in the Adjuvant Phase in Patients with Resectable Stage II-III Non-small Cell Lung Cancer (NSCLC)
Rare Genitourinary Tumors
Abstract #TPS4627
June 2, 2024
9:00 AM – 12 PM CDT
(TiP)
SMART: A Phase II Study of Sacituzumab Govitecan (SG) with or without Atezolizumab Immunotherapy in Rare Genitourinary (GU) Tumors such as Small Cell, Adenocarcinoma, and Squamous Cell Bladder/Urinary Tract Cancer, Renal Medullary Carcinoma (RMC) and Penile Cancer*
Solid Tumors
Abstract #3029
June 1, 2024
9:00 AM – 12:00 PM CDT (Poster)
Pooled Safety Analysis of Sacituzumab Govitecan (SG) in Multiple Solid Tumor Types
Thyroid Cancer
Abstract #TPS6130
June 2, 2024
9:00 AM – 12:00 PM CDT
(TiP)
Sacituzumab Govitecan in Patients with Advanced or Metastatic Radioactive-iodine Refractory Thyroid Carcinoma: The Phase 2 SETHY, GETNE-T2318 Trial
Urothelial Cancer
Abstract #e16500
May 23, 2024
4:00 PM CDT
(Online Publication Only)
Treatment Patterns of Metastatic Urothelial Cancer in the United States
Abstract #TPS4618
June 2, 2024
9:00 AM – 12:00 PM CDT (TiP)
Sacituzumab Govitecan (SG) plus Enfortumab Vedotin (EV) for Metastatic Urothelial Carcinoma (mUC) Treatment Experienced (DAD) and with Pembrolizumab (P) in Treatment Naïve UC (DAD-IO)**
*Collaborative study with Dana-Farber Cancer Institute
**Collaborative study
Domvanalimab, zimberelimab and etrumadenant are investigational molecules. Neither Gilead nor Arcus has received approval from any regulatory authority for any use of these molecules, and their safety and efficacy for the treatment of gastrointestinal and lung cancers have not been established.
Trodelvy has not been approved by any regulatory agency for the treatment of metastatic NSCLC. Its safety and efficacy have not been established for this indication. Trodelvy has a Boxed Warning for severe or life-threatening neutropenia and severe diarrhea; please see below for the approved U.S. Indication and additional Important Safety Information.
About Trodelvy
Trodelvy® (sacituzumab govitecan-hziy) is a first-in-class Trop-2-directed antibody-drug conjugate. Trop-2 is a cell surface antigen highly expressed in multiple tumor types, including in more than 90% of breast, bladder and lung cancers. Trodelvy is intentionally designed with a proprietary hydrolyzable linker attached to SN-38, a topoisomerase I inhibitor payload. This unique combination delivers potent activity to both Trop-2 expressing cells and the tumor microenvironment through a bystander effect.
Trodelvy is approved in almost 50 countries, with multiple additional regulatory reviews underway worldwide, for the treatment of adult patients with unresectable locally advanced or metastatic triple-negative breast cancer (TNBC) who have received two or more prior systemic therapies, at least one of them for metastatic disease.
Trodelvy is also approved to treat certain patients with pre-treated HR+/HER2- metastatic breast cancer in Australia, Brazil, Canada, the European Union, Israel, United Arab Emirates and the United States. In the U.S., Trodelvy has an accelerated approval for treatment of certain patients with second-line metastatic urothelial cancer; see below for full indication statements.
Trodelvy is being explored for potential investigational use in other TNBC, HR+/HER2- and metastatic UC populations, as well as a range of tumor types where Trop-2 is highly expressed, including metastatic non-small cell lung cancer (NSCLC), head and neck cancer, gynecological cancer, and gastrointestinal cancers.
U.S. Indications for Trodelvy
In the United States, Trodelvy is indicated for the treatment of adult patients with:
Unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC) who have received two or more prior systemic therapies, at least one of them for metastatic disease.
Unresectable locally advanced or metastatic hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative (IHC 0, IHC 1+ or IHC 2+/ISH–) breast cancer who have received endocrine-based therapy and at least two additional systemic therapies in the metastatic setting.
Locally advanced or metastatic urothelial cancer (mUC) who have previously received a platinum-containing chemotherapy and either programmed death receptor-1 (PD-1) or programmed death-ligand 1 (PD-L1) inhibitor. This indication is approved under accelerated approval based on tumor response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
U.S. Important Safety Information for Trodelvy
BOXED WARNING: NEUTROPENIA AND DIARRHEA
Severe or life-threatening neutropenia may occur. Withhold Trodelvy for absolute neutrophil count below 1500/mm3 or neutropenic fever. Monitor blood cell counts periodically during treatment. Consider G-CSF for secondary prophylaxis. Initiate anti-infective treatment in patients with febrile neutropenia without delay.
Severe diarrhea may occur. Monitor patients with diarrhea and give fluid and electrolytes as needed. At the onset of diarrhea, evaluate for infectious causes and, if negative, promptly initiate loperamide. If severe diarrhea occurs, withhold Trodelvy until resolved to ≤Grade 1 and reduce subsequent doses.
CONTRAINDICATIONS
Severe hypersensitivity reaction to Trodelvy.
WARNINGS AND PRECAUTIONS
Neutropenia: Severe, life-threatening, or fatal neutropenia can occur and may require dose modification. Neutropenia occurred in 64% of patients treated with Trodelvy. Grade 3-4 neutropenia occurred in 49% of patients. Febrile neutropenia occurred in 6%. Neutropenic colitis occurred in 1.4%. Withhold Trodelvy for absolute neutrophil count below 1500/mm3 on Day 1 of any cycle or neutrophil count below 1000/mm3 on Day 8 of any cycle. Withhold Trodelvy for neutropenic fever. Administer G-CSF as clinically indicated or indicated in Table 1 of USPI.
Diarrhea: Diarrhea occurred in 64% of all patients treated with Trodelvy. Grade 3-4 diarrhea occurred in 11% of patients. One patient had intestinal perforation following diarrhea. Diarrhea that led to dehydration and subsequent acute kidney injury occurred in 0.7% of all patients. Withhold Trodelvy for Grade 3-4 diarrhea and resume when resolved to ≤Grade 1. At onset, evaluate for infectious causes and if negative, promptly initiate loperamide, 4 mg initially followed by 2 mg with every episode of diarrhea for a maximum of 16 mg daily. Discontinue loperamide 12 hours after diarrhea resolves. Additional supportive measures (e.g., fluid and electrolyte substitution) may also be employed as clinically indicated. Patients who exhibit an excessive cholinergic response to treatment can receive appropriate premedication (e.g., atropine) for subsequent treatments.
Hypersensitivity and Infusion-Related Reactions: Serious hypersensitivity reactions including life-threatening anaphylactic reactions have occurred with Trodelvy. Severe signs and symptoms included cardiac arrest, hypotension, wheezing, angioedema, swelling, pneumonitis, and skin reactions. Hypersensitivity reactions within 24 hours of dosing occurred in 35% of patients. Grade 3-4 hypersensitivity occurred in 2% of patients. The incidence of hypersensitivity reactions leading to permanent discontinuation of Trodelvy was 0.2%. The incidence of anaphylactic reactions was 0.2%. Pre-infusion medication is recommended. Have medications and emergency equipment to treat such reactions available for immediate use. Observe patients closely for hypersensitivity and infusion-related reactions during each infusion and for at least 30 minutes after completion of each infusion. Permanently discontinue Trodelvy for Grade 4 infusion-related reactions.
Nausea and Vomiting: Nausea occurred in 64% of all patients treated with Trodelvy and Grade 3-4 nausea occurred in 3% of these patients. Vomiting occurred in 35% of patients and Grade 3-4 vomiting occurred in 2% of these patients. Premedicate with a two or three drug combination regimen (e.g., dexamethasone with either a 5-HT3 receptor antagonist or an NK1 receptor antagonist as well as other drugs as indicated) for prevention of chemotherapy-induced nausea and vomiting (CINV). Withhold Trodelvy doses for Grade 3 nausea or Grade 3-4 vomiting and resume with additional supportive measures when resolved to Grade ≤1. Additional antiemetics and other supportive measures may also be employed as clinically indicated. All patients should be given take-home medications with clear instructions for prevention and treatment of nausea and vomiting.
Increased Risk of Adverse Reactions in Patients with Reduced UGT1A1 Activity: Patients homozygous for the uridine diphosphate-glucuronosyl transferase 1A1 (UGT1A1)*28 allele are at increased risk for neutropenia, febrile neutropenia, and anemia and may be at increased risk for other adverse reactions with Trodelvy. The incidence of Grade 3-4 neutropenia was 58% in patients homozygous for the UGT1A1*28, 49% in patients heterozygous for the UGT1A1*28 allele, and 43% in patients homozygous for the wild-type allele. The incidence of Grade 3-4 anemia was 21% in patients homozygous for the UGT1A1*28 allele, 10% in patients heterozygous for the UGT1A1*28 allele, and 9% in patients homozygous for the wild-type allele. Closely monitor patients with known reduced UGT1A1 activity for adverse reactions. Withhold or permanently discontinue Trodelvy based on clinical assessment of the onset, duration and severity of the observed adverse reactions in patients with evidence of acute early-onset or unusually severe adverse reactions, which may indicate reduced UGT1A1 function.
Embryo-Fetal Toxicity: Based on its mechanism of action, Trodelvy can cause teratogenicity and/or embryo-fetal lethality when administered to a pregnant woman. Trodelvy contains a genotoxic component, SN-38, and targets rapidly dividing cells. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with Trodelvy and for 6 months after the last dose. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with Trodelvy and for 3 months after the last dose.
ADVERSE REACTIONS
In the pooled safety population, the most common (≥ 25%) adverse reactions including laboratory abnormalities were decreased leukocyte count (84%), decreased neutrophil count (75%), decreased hemoglobin (69%), diarrhea (64%), nausea (64%), decreased lymphocyte count (63%), fatigue (51%), alopecia (45%), constipation (37%), increased glucose (37%), decreased albumin (35%), vomiting (35%), decreased appetite (30%), decreased creatinine clearance (28%), increased alkaline phosphatase (28%), decreased magnesium (27%), decreased potassium (26%), and decreased sodium (26%).
In the ASCENT study (locally advanced or metastatic triple-negative breast cancer), the most common adverse reactions (incidence ≥25%) were fatigue, diarrhea, nausea, alopecia, constipation, vomiting, abdominal pain, and decreased appetite. The most frequent serious adverse reactions (SAR) (>1%) were neutropenia (7%), diarrhea (4%), and pneumonia (3%). SAR were reported in 27% of patients, and 5% discontinued therapy due to adverse reactions. The most common Grade 3-4 lab abnormalities (incidence ≥25%) in the ASCENT study were reduced neutrophils, leukocytes, and lymphocytes.
In the TROPiCS-02 study (locally advanced or metastatic HR-positive, HER2-negative breast cancer), the most common adverse reactions (incidence ≥25%) were diarrhea, fatigue, nausea, alopecia, and constipation. The most frequent serious adverse reactions (SAR) (>1%) were diarrhea (5%), febrile neutropenia (4%), neutropenia (3%), abdominal pain, colitis, neutropenic colitis, pneumonia, and vomiting (each 2%). SAR were reported in 28% of patients, and 6% discontinued therapy due to adverse reactions. The most common Grade 3-4 lab abnormalities (incidence ≥25%) in the TROPiCS-02 study were reduced neutrophils and leukocytes.
In the TROPHY study (locally advanced or metastatic urothelial cancer), the most common adverse reactions (incidence ≥25%) were diarrhea, fatigue, nausea, any infection, alopecia, decreased appetite, constipation, vomiting, rash, and abdominal pain. The most frequent serious adverse reactions (SAR) (≥5%) were infection (18%), neutropenia (12%, including febrile neutropenia in 10%), acute kidney injury (6%), urinary tract infection (6%), and sepsis or bacteremia (5%). SAR were reported in 44% of patients, and 10% discontinued due to adverse reactions. The most common Grade 3-4 lab abnormalities (incidence ≥25%) in the TROPHY study were reduced neutrophils, leukocytes, and lymphocytes.
DRUG INTERACTIONS
UGT1A1 Inhibitors: Concomitant administration of Trodelvy with inhibitors of UGT1A1 may increase the incidence of adverse reactions due to potential increase in systemic exposure to SN-38. Avoid administering UGT1A1 inhibitors with Trodelvy.
UGT1A1 Inducers: Exposure to SN-38 may be reduced in patients concomitantly receiving UGT1A1 enzyme inducers. Avoid administering UGT1A1 inducers with Trodelvy.
Please see full Prescribing Information, including BOXED WARNING.
About Tecartus
Please see full FDA Prescribing Information, including BOXED WARNING and Medication Guide.
Tecartus is a CD19-directed genetically modified autologous T cell immunotherapy indicated for the treatment of:
Adult patients with relapsed or refractory mantle cell lymphoma (MCL).
This indication is approved under accelerated approval based on overall response rate and durability of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
Adult patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL).
U.S. IMPORTANT SAFETY INFORMATION
BOXED WARNING: CYTOKINE RELEASE SYNDROME, NEUROLOGIC TOXICITIES, and SECONDARY HEMATOLOGICAL MALIGNANCIES
Cytokine Release Syndrome (CRS), including life-threatening reactions, occurred in patients receiving Tecartus. Do not administer Tecartus to patients with active infection or inflammatory disorders. Treat severe or life-threatening CRS with tocilizumab or tocilizumab and corticosteroids.
Neurologic toxicities, including life-threatening reactions, occurred in patients receiving Tecartus, including concurrently with CRS or after CRS resolution. Monitor for neurologic toxicities after treatment with Tecartus. Provide supportive care and/or corticosteroids as needed.
T-cell malignancies have occurred following treatment of hematologic malignancies with BCMA- and CD19-directed genetically modified autologous T-cell immunotherapies.
Tecartus is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the Yescarta and Tecartus REMS Program.
Ensure that a minimum of two doses of tocilizumab are available for each patient prior to infusion of Tecartus. Following infusion, monitor patients for signs and symptoms of CRS daily for at least seven days at the certified healthcare facility, and for four weeks thereafter. Counsel patients to seek immediate medical attention should signs or symptoms of CRS occur at any time. At the first sign of CRS, institute treatment with supportive care, tocilizumab, or tocilizumab and corticosteroids as indicated.
Neurologic Events, including those that were fatal or life-threatening, occurred following treatment with Tecartus. Neurologic events occurred in 87% (68/78) of patients with ALL, including ≥ Grade 3 in 35% of patients. The median time to onset for neurologic events was seven days (range: 1 to 51 days) with a median duration of 15 days (range: 1 to 397 days) in patients with ALL. For patients with MCL, 54 (66%) patients experienced CRS before the onset of neurological events. Five (6%) patients did not experience CRS with neurologic events and eight patients (10%) developed neurological events after the resolution of CRS. Neurologic events resolved for 119 out of 134 (89%) patients treated with Tecartus. Nine patients (three patients with MCL and six patients with ALL) had ongoing neurologic events at the time of death. For patients with ALL, neurologic events occurred before, during, and after CRS in 4 (5%), 57 (73%), and 8 (10%) of patients; respectively. Three patients (4%) had neurologic events without CRS. The onset of neurologic events can be concurrent with CRS, following resolution of CRS or in the absence of CRS.
The most common neurologic events (>10%) were similar in MCL and ALL and included encephalopathy (57%), headache (37%), tremor (34%), confusional state (26%), aphasia (23%), delirium (17%), dizziness (15%), anxiety (14%), and agitation (12%). Serious events including encephalopathy, aphasia, confusional state, and seizures occurred after treatment with Tecartus.
Monitor patients daily for at least seven days for patients with MCL and at least 14 days for patients with ALL at the certified healthcare facility and for four weeks following infusion for signs and symptoms of neurologic toxicities and treat promptly.
REMS Program: Because of the risk of CRS and neurologic toxicities, Tecartus is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the Yescarta and Tecartus REMS Program which requires that:
Healthcare facilities that dispense and administer Tecartus must be enrolled and comply with the REMS requirements. Certified healthcare facilities must have on-site, immediate access to tocilizumab, and ensure that a minimum of two doses of tocilizumab are available for each patient for infusion within two hours after Tecartus infusion, if needed for treatment of CRS.
Certified healthcare facilities must ensure that healthcare providers who prescribe, dispense, or administer Tecartus are trained in the management of CRS and neurologic toxicities. Further information is available at or 1-844-454-KITE (5483).
Hypersensitivity Reactions: Serious hypersensitivity reactions, including anaphylaxis, may occur due to dimethyl sulfoxide (DMSO) or residual gentamicin in Tecartus.
Severe Infections: Severe or life-threatening infections occurred in patients after Tecartus infusion. Infections (all grades) occurred in 56% (46/82) of patients with MCL and 44% (34/78) of patients with ALL. Grade 3 or higher infections, including bacterial, viral, and fungal infections, occurred in 30% of patients with ALL and MCL. Tecartus should not be administered to patients with clinically significant active systemic infections. Monitor patients for signs and symptoms of infection before and after Tecartus infusion and treat appropriately. Administer prophylactic antimicrobials according to local guidelines.
Febrile neutropenia was observed in 6% of patients with MCL and 35% of patients with ALL after Tecartus infusion and may be concurrent with CRS. The febrile neutropenia in 27 (35%) of patients with ALL includes events of “febrile neutropenia” (11 (14%)) plus the concurrent events of “fever” and “neutropenia” (16 (21%)). In the event of febrile neutropenia, evaluate for infection and manage with broad spectrum antibiotics, fluids, and other supportive care as medically indicated.
In immunosuppressed patients, life-threatening and fatal opportunistic infections have been reported. The possibility of rare infectious etiologies (e.g., fungal and viral infections such as HHV-6 and progressive multifocal leukoencephalopathy) should be considered in patients with neurologic events and appropriate diagnostic evaluations should be performed.
Hepatitis B virus (HBV) reactivation, in some cases resulting in fulminant hepatitis, hepatic failure, and death, can occur in patients treated with drugs directed against B cells. Perform screening for HBV, HCV, and HIV in accordance with clinical guidelines before collection of cells for manufacturing.
Prolonged Cytopenias: Patients may exhibit cytopenias for several weeks following lymphodepleting chemotherapy and Tecartus infusion. In patients with MCL, Grade 3 or higher cytopenias not resolved by Day 30 following Tecartus infusion occurred in 55% (45/82) of patients and included thrombocytopenia (38%), neutropenia (37%), and anemia (17%). In patients with ALL who were responders to Tecartus treatment, Grade 3 or higher cytopenias not resolved by Day 30 following Tecartus infusion occurred in 20% (7/35) of the patients and included neutropenia (12%) and thrombocytopenia (12%); Grade 3 or higher cytopenias not resolved by Day 60 following Tecartus infusion occurred in 11% (4/35) of the patients and included neutropenia (9%) and thrombocytopenia (6%). Monitor blood counts after Tecartus infusion.
Hypogammaglobulinemia: B cell aplasia and hypogammaglobulinemia can occur in patients receiving treatment with Tecartus. Hypogammaglobulinemia was reported in 16% (13/82) of patients with MCL and 9% (7/78) of patients with ALL. Monitor immunoglobulin levels after treatment with Tecartus and manage using infection precautions, antibiotic prophylaxis, and immunoglobulin replacement.
The safety of immunization with live viral vaccines during or following Tecartus treatment has not been studied. Vaccination with live virus vaccines is not recommended for at least six weeks prior to the start of lymphodepleting chemotherapy, during Tecartus treatment, and until immune recovery following treatment with Tecartus.
Secondary Malignancies: Patients treated with TECARTUS may develop secondary malignancies. T-cell malignancies have occurred following treatment of hematologic malignancies with BCMA- and CD19-directed genetically modified autologous T-cell immunotherapies. Mature T-cell malignancies, including CAR-positive tumors, may present as soon as weeks following infusions, and may include fatal outcomes.
Monitor life-long for secondary malignancies. In the event that a secondary malignancy occurs, contact Kite at 1-844-454-KITE (5483) to obtain instructions on patient samples to collect for testing.
Effects on Ability to Drive and Use Machines: Due to the potential for neurologic events, including altered mental status or seizures, patients are at risk for altered or decreased consciousness or coordination in the 8 weeks following Tecartus infusion. Advise patients to refrain from driving and engaging in hazardous activities, such as operating heavy or potentially dangerous machinery, during this period.
Adverse Reactions: The most common non-laboratory adverse reactions (≥ 20%) were fever, cytokine release syndrome, hypotension, encephalopathy, tachycardia, nausea, chills, headache, fatigue, febrile neutropenia, diarrhea, musculoskeletal pain, hypoxia, rash, edema, tremor, infection with pathogen unspecified, constipation, decreased appetite, and vomiting. The most common serious adverse reactions (≥ 2%) were cytokine release syndrome, febrile neutropenia, hypotension, encephalopathy, fever, infection with pathogen unspecified, hypoxia, tachycardia, bacterial infections, respiratory failure, seizure, diarrhea, dyspnea, fungal infections, viral infections, coagulopathy, delirium, fatigue, hemophagocytic lymphohistiocytosis, musculoskeletal pain, edema, and paraparesis.
Please see full Prescribing Information, including BOXED WARNING and Medication Guide.
About Yescarta
Please see full US Prescribing Information, including BOXED WARNING and Medication Guide.
YESCARTA is a CD19-directed genetically modified autologous T cell immunotherapy indicated for the treatment of:
Adult patients with large B-cell lymphoma that is refractory to first-line chemoimmunotherapy or that relapses within 12 months of first-line chemoimmunotherapy.
Adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy, including diffuse large B-cell lymphoma (DLBCL) not otherwise specified, primary mediastinal large B-cell lymphoma, high-grade B-cell lymphoma, and DLBCL arising from follicular lymphoma.
Limitations of Use: YESCARTA is not indicated for the treatment of patients with primary central nervous system lymphoma.
Adult patients with relapsed or refractory follicular lymphoma (FL) after two or more lines of systemic therapy. This indication is approved under accelerated approval based on the response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trial(s).
U.S. IMPORTANT SAFETY INFORMATION
BOXED WARNING: CYTOKINE RELEASE SYNDROME, NEUROLOGIC TOXICITIES, and SECONDARY HEMATOLOGICAL MALIGNANCIES
Cytokine Release Syndrome (CRS), including fatal or life-threatening reactions, occurred in patients receiving YESCARTA. Do not administer YESCARTA to patients with active infection or inflammatory disorders. Treat severe or life-threatening CRS with tocilizumab or tocilizumab and corticosteroids.
Neurologic toxicities, including fatal or life-threatening reactions, occurred in patients receiving YESCARTA, including concurrently with CRS or after CRS resolution. Monitor for neurologic toxicities after treatment with YESCARTA. Provide supportive care and/or corticosteroids as needed.
T-cell malignancies have occurred following treatment of hematologic malignancies with BCMA-and CD19-directed genetically modified autologous T cell immunotherapies, including YESCARTA.
YESCARTA is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the YESCARTA and TECARTUS REMS Program.
CYTOKINE RELEASE SYNDROME (CRS)
CRS, including fatal or life-threatening reactions, occurred following treatment with YESCARTA. CRS occurred in 90% (379/422) of patients with non-Hodgkin lymphoma (NHL), including ≥ Grade 3 CRS in 9%. CRS occurred in 93% (256/276) of patients with large B-cell lymphoma (LBCL), including ≥ Grade 3 in 9%. Among patients with LBCL who died after receiving YESCARTA, 4 had ongoing CRS events at the time of death. For patients with LBCL in ZUMA-1, the median time to onset of CRS was 2 days following infusion (range: 1-12 days) and the median duration was 7 days (range: 2-58 days). For patients with LBCL in ZUMA-7, the median time to onset of CRS was 3 days following infusion (range: 1-10 days) and the median duration was 7 days (range: 2-43 days).
CRS occurred in 84% (123/146) of patients with indolent non-Hodgkin lymphoma (iNHL) in ZUMA-5, including ≥ Grade 3 CRS in 8%. Among patients with iNHL who died after receiving YESCARTA, 1 patient had an ongoing CRS event at the time of death. The median time to onset of CRS was 4 days (range: 1-20 days) and median duration was 6 days (range: 1-27 days) for patients with iNHL.
Key manifestations of CRS (≥ 10%) in all patients combined included fever (85%), hypotension (40%), tachycardia (32%), chills (22%), hypoxia (20%), headache (15%), and fatigue (12%). Serious events that may be associated with CRS include, cardiac arrhythmias (including atrial fibrillation and ventricular tachycardia), renal insufficiency, cardiac failure, respiratory failure, cardiac arrest, capillary leak syndrome, multi-organ failure, and hemophagocytic lymphohistiocytosis/macrophage activation syndrome.
The impact of tocilizumab and/or corticosteroids on the incidence and severity of CRS was assessed in 2 subsequent cohorts of LBCL patients in ZUMA-1. Among patients who received tocilizumab and/or corticosteroids for ongoing Grade 1 events, CRS occurred in 93% (38/41), including 2% (1/41) with Grade 3 CRS; no patients experienced a Grade 4 or 5 event. The median time to onset of CRS was 2 days (range: 1-8 days) and the median duration of CRS was 7 days (range: 2-16 days). Prophylactic treatment with corticosteroids was administered to a cohort of 39 patients for 3 days beginning on the day of infusion of YESCARTA. Thirty-one of the 39 patients (79%) developed CRS and were managed with tocilizumab and/or therapeutic doses of corticosteroids with no patients developing ≥ Grade 3 CRS. The median time to onset of CRS was 5 days (range: 1-15 days) and the median duration of CRS was 4 days (range: 1-10 days). Although there is no known mechanistic explanation, consider the risk and benefits of prophylactic corticosteroids in the context of pre-existing comorbidities for the individual patient and the potential for the risk of Grade 4 and prolonged neurologic toxicities.
Ensure that 2 doses of tocilizumab are available prior to YESCARTA infusion. Monitor patients for signs and symptoms of CRS at least daily for 7 days at the certified healthcare facility, and for 4 weeks thereafter. Counsel patients to seek immediate medical attention should signs or symptoms of CRS occur at any time. At the first sign of CRS, institute treatment with supportive care, tocilizumab, or tocilizumab and corticosteroids as indicated.
NEUROLOGIC TOXICITIES
Neurologic toxicities (including immune effector cell-associated neurotoxicity syndrome) that were fatal or life-threatening occurred. Neurologic toxicities occurred in 78% (330/422) of all patients with NHL receiving YESCARTA, including ≥ Grade 3 in 25%. Neurologic toxicities occurred in 87% (94/108) of patients with LBCL in ZUMA-1, including ≥ Grade 3 in 31% and in 74% (124/168) of patients in ZUMA-7 including ≥ Grade 3 in 25%. The median time to onset was 4 days (range: 1-43 days) and the median duration was 17 days for patients with LBCL in ZUMA-1. The median time to onset for neurologic toxicity was 5 days (range:1- 133 days) and the median duration was 15 days in patients with LBCL in ZUMA-7. Neurologic toxicities occurred in 77% (112/146) of patients with iNHL, including ≥ Grade 3 in 21%. The median time to onset was 6 days (range: 1-79 days) and the median duration was 16 days. Ninety-eight percent of all neurologic toxicities in patients with LBCL and 99% of all neurologic toxicities in patients with iNHL occurred within the first 8 weeks of YESCARTA infusion. Neurologic toxicities occurred within the first 7 days of infusion for 87% of affected patients with LBCL and 74% of affected patients with iNHL.
The most common neurologic toxicities (≥ 10%) in all patients combined included encephalopathy (50%), headache (43%), tremor (29%), dizziness (21%), aphasia (17%), delirium (15%), and insomnia (10%). Prolonged encephalopathy lasting up to 173 days was noted. Serious events, including aphasia, leukoencephalopathy, dysarthria, lethargy, and seizures occurred. Fatal and serious cases of cerebral edema and encephalopathy, including late-onset encephalopathy, have occurred.
The impact of tocilizumab and/or corticosteroids on the incidence and severity of neurologic toxicities was assessed in 2 subsequent cohorts of LBCL patients in ZUMA-1. Among patients who received corticosteroids at the onset of Grade 1 toxicities, neurologic toxicities occurred in 78% (32/41), and 20% (8/41) had Grade 3 neurologic toxicities; no patients experienced a Grade 4 or 5 event. The median time to onset of neurologic toxicities was 6 days (range: 1-93 days) with a median duration of 8 days (range: 1-144 days). Prophylactic treatment with corticosteroids was administered to a cohort of 39 patients for 3 days beginning on the day of infusion of YESCARTA. Of those patients, 85% (33/39) developed neurologic toxicities, 8% (3/39) developed Grade 3, and 5% (2/39) developed Grade 4 neurologic toxicities. The median time to onset of neurologic toxicities was 6 days (range: 1-274 days) with a median duration of 12 days (range: 1-107 days). Prophylactic corticosteroids for management of CRS and neurologic toxicities may result in a higher grade of neurologic toxicities or prolongation of neurologic toxicities, delay the onset of and decrease the duration of CRS.
Monitor patients for signs and symptoms of neurologic toxicities at least daily for 7 days at the certified healthcare facility, and for 4 weeks thereafter, and treat promptly.
REMS
Because of the risk of CRS and neurologic toxicities, YESCARTA is available only through a restricted program called the YESCARTA and TECARTUS REMS Program which requires that: Healthcare facilities that dispense and administer YESCARTA must be enrolled and comply with the REMS requirements and must have on-site, immediate access to a minimum of 2 doses of tocilizumab for each patient for infusion within 2 hours after YESCARTA infusion, if needed for treatment of CRS. Certified healthcare facilities must ensure that healthcare providers who prescribe, dispense, or administer YESCARTA are trained in the management of CRS and neurologic toxicities. Further information is available at or 1-844-454-KITE (5483).
HYPERSENSITIVITY REACTIONS
Allergic reactions, including serious hypersensitivity reactions or anaphylaxis, may occur with the infusion of YESCARTA.
SERIOUS INFECTIONS
Severe or life-threatening infections occurred. Infections (all grades) occurred in 45% of patients with NHL; ≥ Grade 3 infections occurred in 17% of patients, including ≥ Grade 3 infections with an unspecified pathogen in 12%, bacterial infections in 5%, viral infections in 3%, and fungal infections in 1%. YESCARTA should not be administered to patients with clinically significant active systemic infections. Monitor patients for signs and symptoms of infection before and after infusion and treat appropriately. Administer prophylactic antimicrobials according to local guidelines.
Febrile neutropenia was observed in 36% of all patients with NHL and may be concurrent with CRS. In the event of febrile neutropenia, evaluate for infection and manage with broad-spectrum antibiotics, fluids, and other supportive care as medically indicated.
In immunosuppressed patients, including those who have received YESCARTA, life-threatening and fatal opportunistic infections including disseminated fungal infections (e.g., candida sepsis and aspergillus infections) and viral reactivation (e.g., human herpes virus-6 [HHV-6] encephalitis and JC virus progressive multifocal leukoencephalopathy [PML]) have been reported. The possibility of HHV-6 encephalitis and PML should be considered in immunosuppressed patients with neurologic events and appropriate diagnostic evaluations should be performed.
Hepatitis B virus (HBV) reactivation, in some cases resulting in fulminant hepatitis, hepatic failure, and death, can occur in patients treated with drugs directed against B cells, including YESCARTA. Perform screening for HBV, HCV, and HIV in accordance with clinical guidelines before collection of cells for manufacturing.
PROLONGED CYTOPENIAS
Patients may exhibit cytopenias for several weeks following lymphodepleting chemotherapy and YESCARTA infusion. ≥ Grade 3 cytopenias not resolved by Day 30 following YESCARTA infusion occurred in 39% of all patients with NHL and included neutropenia (33%), thrombocytopenia (13%), and anemia (8%). Monitor blood counts after infusion.
HYPOGAMMAGLOBULINEMIA
B-cell aplasia and hypogammaglobulinemia can occur. Hypogammaglobulinemia was reported as an adverse reaction in 14% of all patients with NHL. Monitor immunoglobulin levels after treatment and manage using infection precautions, antibiotic prophylaxis, and immunoglobulin replacement. The safety of immunization with live viral vaccines during or following YESCARTA treatment has not been studied. Vaccination with live virus vaccines is not recommended for at least 6 weeks prior to the start of lymphodepleting chemotherapy, during YESCARTA treatment, and until immune recovery following treatment.
SECONDARY MALIGNANCIES
Patients treated with YESCARTA may develop secondary malignancies. T-cell malignancies have occurred following treatment of hematologic malignancies with BCMA- and CD19-directed genetically modified autologous T-cell immunotherapies, including YESCARTA. Mature T-cell malignancies, including CAR-positive tumors, may present as soon as weeks following infusion, and may include fatal outcomes.
Monitor life-long for secondary malignancies. In the event that a secondary malignancy occurs, contact Kite at 1-844-454-KITE (5483) to obtain instructions on patient samples to collect for testing.
EFFECTS ON ABILITY TO DRIVE AND USE MACHINES
Due to the potential for neurologic events, including altered mental status or seizures, patients are at risk for altered or decreased consciousness or coordination in the 8 weeks following YESCARTA infusion. Advise patients to refrain from driving and engaging in hazardous occupations or activities, such as operating heavy or potentially dangerous machinery, during this initial period.
ADVERSE REACTIONS
The most common non-laboratory adverse reactions (incidence ≥ 20%) in patients with LBCL in ZUMA-7 included fever, CRS, fatigue, hypotension, encephalopathy, tachycardia, diarrhea, headache, musculoskeletal pain, nausea, febrile neutropenia, chills, cough, infection with an unspecified pathogen, dizziness, tremor, decreased appetite, edema, hypoxia, abdominal pain, aphasia, constipation, and vomiting.
The most common adverse reactions (incidence ≥ 20%) in patients with LBCL in ZUMA-1 included CRS, fever, hypotension, encephalopathy, tachycardia, fatigue, headache, decreased appetite, chills, diarrhea, febrile neutropenia, infections with an unspecified, nausea, hypoxia, tremor, cough, vomiting, dizziness, constipation, and cardiac arrhythmias.
The most common non-laboratory adverse reactions (incidence ≥ 20%) in patients with iNHL in ZUMA-5 included fever, CRS, hypotension, encephalopathy, fatigue, headache, infections with an unspecified, tachycardia, febrile neutropenia, musculoskeletal pain, nausea, tremor, chills, diarrhea, constipation, decreased appetite, cough, vomiting, hypoxia, arrhythmia, and dizziness.
About Domvanalimab
Domvanalimab is the first and most clinically advanced Fc-silent investigational monoclonal antibody that is specifically designed with Fc-silent properties to block and bind to the T-cell immunoreceptor with Ig and ITIM domains (TIGIT), a checkpoint receptor on immune cells that acts as a brake on the anticancer immune response. By binding to TIGIT with Fc-silent properties, domvanalimab is believed to work by freeing up immune-activating pathways and activate immune cells to attack and kill cancer cells without depleting the peripheral regulatory T cells important in avoiding immune-related toxicity.
Combined inhibition of both TIGIT and programmed cell death protein-1 (PD-1) is believed to significantly enhance immune cell activation, as these checkpoint receptors play distinct, complementary roles in anti-tumor activity. Domvanalimab is being evaluated in combination with anti-PD-1 monoclonal antibodies, including zimberelimab, as well as other investigational cancer immunotherapies and A2a/A2b adenosine receptor antagonist etrumadenant, in multiple ongoing and planned early and late-stage clinical studies in various tumor types.
About Zimberelimab
Zimberelimab is an anti-programmed cell death protein-1 (PD-1) monoclonal antibody that binds PD-1, with the goal of restoring the antitumor activity of T cells. Zimberelimab has demonstrated high affinity, selectivity and potency in various tumor types.
Zimberelimab is being evaluated in the U.S. and globally as a foundational PD-1 treatment option in multiple ongoing and planned early and late-stage clinical studies in combination with other immunotherapies, including investigational Fc-silent anti-TIGIT monoclonal antibody domvanalimab and A2a/A2b adenosine receptor antagonist etrumadenant.
Guangzhou Gloria Biosciences Co. Ltd., who holds commercialization rights for zimberelimab in greater China, has obtained approval for zimberelimab for the treatment of recurrent or metastatic cervical cancer and for relapsed or refractory classical Hodgkin's lymphoma. Zimberelimab is not approved for any use in the U.S. or other regions outside of China. Gloria conducts its development and commercialization activities independent of Arcus and Gilead.
About Etrumadenant
Etrumadenant is an investigational small molecule, selective dual antagonist of the A2a and A2b receptors designed to prevent adenosine-mediated immunosuppression. Adenosine elicits its immunosuppressive effects within the tumor microenvironment by binding and activating adenosine-specific receptors expressed on the surface of tumor-infiltrating immune cells, which can help cancer cells evade host antitumor immunity. Once etrumadenant binds to the A2a and A2b receptors and blocks the immunosuppressive effects of adenosine, activation of antitumor immune cells may be restored, which could result in tumor cell death.
Etrumadenant is being evaluated in combination with other cancer immunotherapies, including the investigational Fc-silent anti-TIGIT monoclonal antibody domvanalimab and PD-1 inhibitor zimberelimab, in certain types of non-small cell lung and colorectal cancers.
About Gilead Sciences
Gilead Sciences, Inc. is a biopharmaceutical company that has pursued and achieved breakthroughs in medicine for more than three decades, with the goal of creating a healthier world for all people. The company is committed to advancing innovative medicines to prevent and treat life-threatening diseases, including HIV, viral hepatitis, COVID-19, and cancer. Gilead operates in more than 35 countries worldwide, with headquarters in Foster City, California.
About Kite
Kite, a Gilead Company, is a global biopharmaceutical company based in Santa Monica, California, focused on cell therapy to treat and potentially cure cancer. As the global cell therapy leader, Kite has treated more patients with CAR T-cell therapy than any other company. Kite has the largest in-house cell therapy manufacturing network in the world, spanning process development, vector manufacturing, clinical trial supply and commercial product manufacturing.
Forward-Looking Statements
This press release includes forward-looking statements, within the meaning of the Private Securities Litigation Reform Act of 1995 that are subject to risks, uncertainties and other factors, including the ability of Gilead and Kite to initiate, progress or complete clinical trials within currently anticipated timelines or at all, and the possibility of unfavorable results from ongoing or additional clinical studies, including those involving Tecartus, Trodelvy, Yescarta, domvanalimab, etrumadenant, and zimberelimab; uncertainties relating to regulatory applications and related filing and approval timelines, including pending or potential applications for indications currently under evaluation; the possibility that Gilead and Kite may make a strategic decision to discontinue development of these programs and, as a result, these programs may never be successfully commercialized for the indications currently under evaluation; and any assumptions underlying any of the foregoing. These and other risks are described in detail in Gilead’s Annual Report on Form 10-K for the year ended December 31, 2023, as filed with the U.S. Securities and Exchange Commission. These risks, uncertainties and other factors could cause actual results to differ materially from those referred to in the forward-looking statements. All statements other than statements of historical fact are statements that could be deemed forward-looking statements. The reader is cautioned that any such forward-looking statements are not guarantees of future performance and involve risks and uncertainties, and is cautioned not to place undue reliance on these forward-looking statements. All forward-looking statements are based on information currently available to Gilead and Kite, and Gilead and Kite assume no obligation and disclaim any intent to update any such forward-looking statements.
Trodelvy, Yescarta, Tecartus, Gilead, the Gilead logo, Kite and the Kite logo are trademarks of Gilead Sciences, Inc., or its related companies.
For more information about Gilead, please visit the company’s website at , follow Gilead on Twitter (@GileadSciences) or call Gilead Public Affairs at 1-800-GILEAD-5 or 1-650-574-3000.
For more information on Kite, please visit the company’s website at . Follow Kite on social media on Twitter (@KitePharma) and LinkedIn.
View source version on businesswire.com:
Contacts
Jacquie Ross, Investors
investor_relations@gilead.com
Meaghan Smith, Media
public_affairs@gilead.com
Anna Padula, Kite Media
apadula@kitepharma.com
Source: Gilead Sciences, Inc.
View this news release online at:
Clinical ResultPhase 2ASCOImmunotherapyPhase 3
100 Deals associated with IT-141
Login to view more data
External Link
| KEGG | Wiki | ATC | Drug Bank |
|---|---|---|---|
| - | - | - |
R&D Status
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Relapsed Solid Neoplasm | Phase 2 | US | 23 Mar 2017 | |
| Solid tumor | Phase 1 | TW | 31 Oct 2023 | |
| Metastatic Colorectal Carcinoma | Phase 1 | US | 01 Jan 2006 | |
| Neoplasms | Phase 1 | - | - | |
| Advanced cancer | Discovery | US | 23 Mar 2017 | |
| Refractory Malignant Solid Neoplasm | Discovery | US | 23 Mar 2017 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
Not Applicable | - | EMICORON + SN-38 | (rmgibznkqb) = dhqsbubffa sopvgneoia (amlmwijhbn ) | Positive | 15 Jul 2016 | ||
(pzhjuoifdb) = ulglawsxel bpvejywrnf (hozjwgbhiv ) View more | |||||||
Not Applicable | - | rsemwwdfra(ysowcowafi) = wxkwcgmbaa cvwitjuvka (mlgwtskwur ) View more | - | 15 Apr 2010 |
Login to view more data
Translational Medicine
Boost your research with our translational medicine data.
login
or
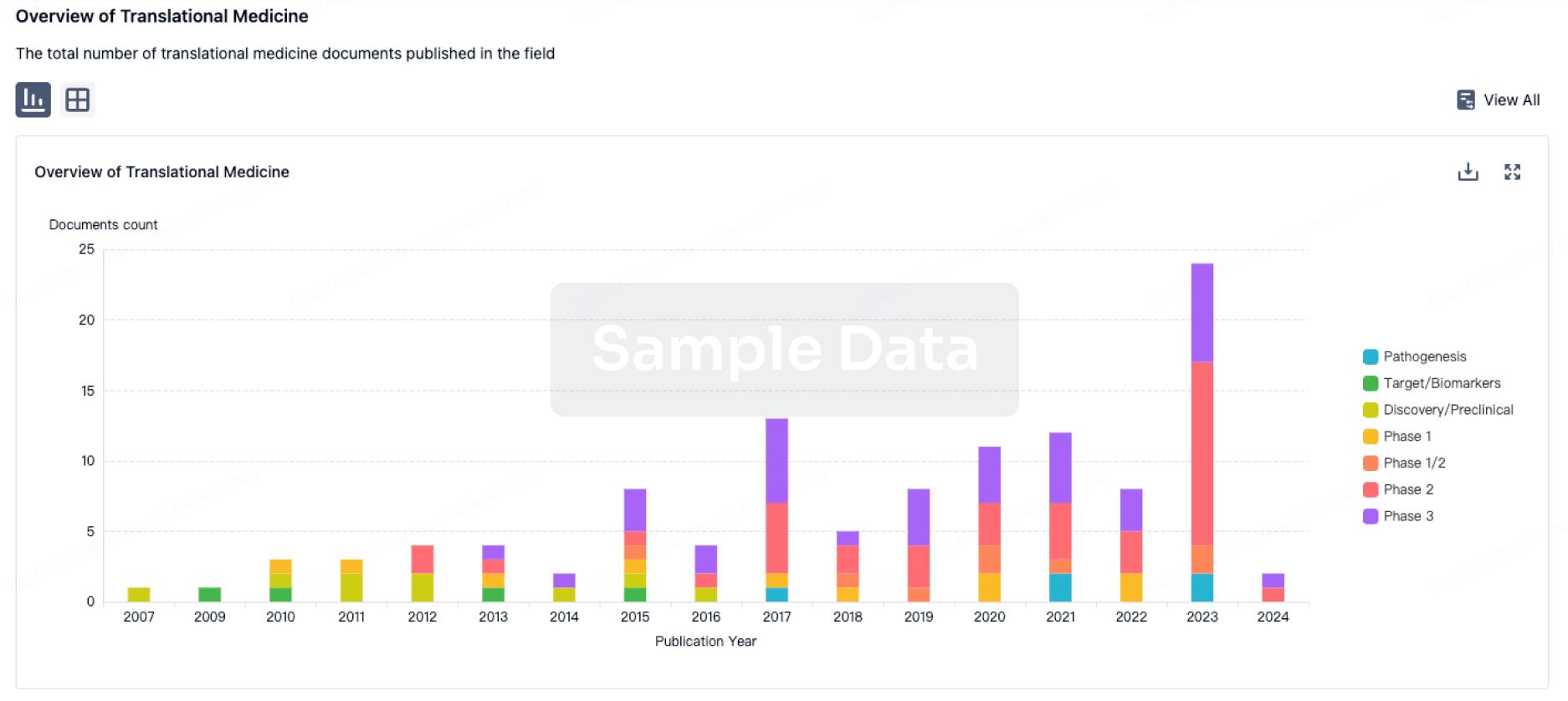
Deal
Boost your decision using our deal data.
login
or
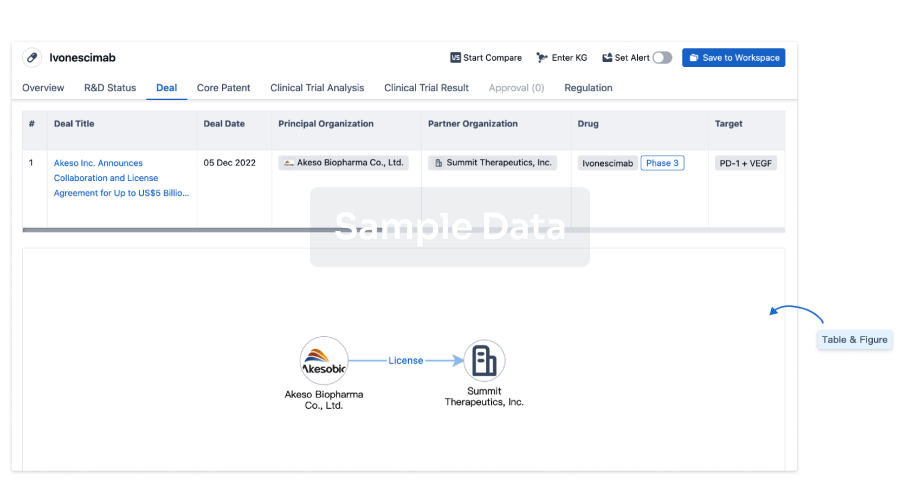
Core Patent
Boost your research with our Core Patent data.
login
or
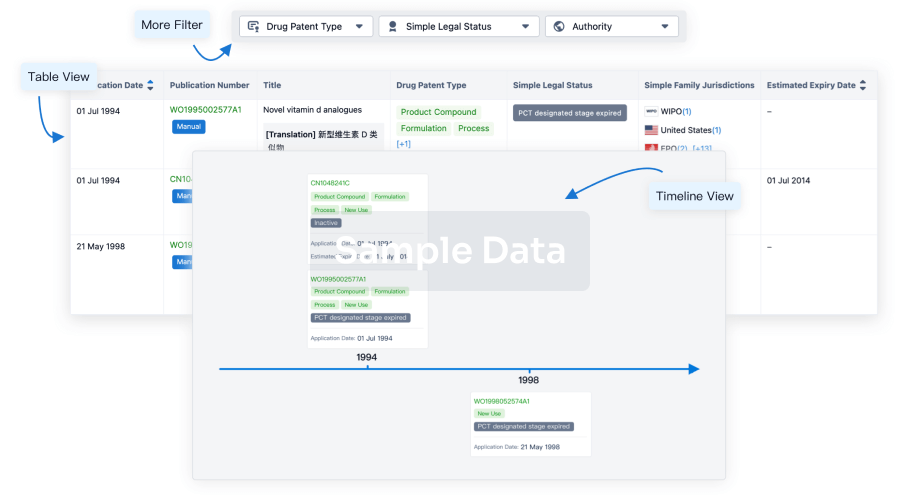
Clinical Trial
Identify the latest clinical trials across global registries.
login
or
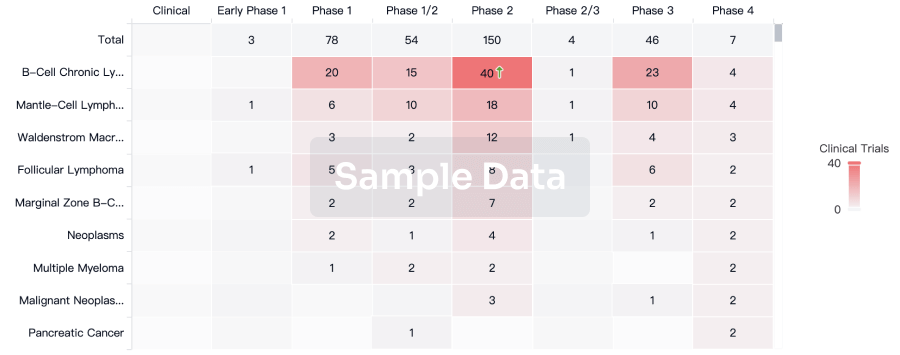
Approval
Accelerate your research with the latest regulatory approval information.
login
or
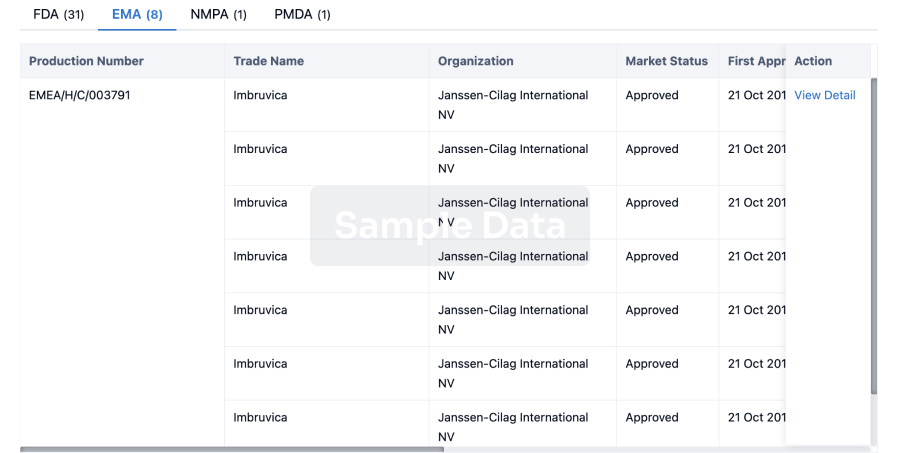
Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or
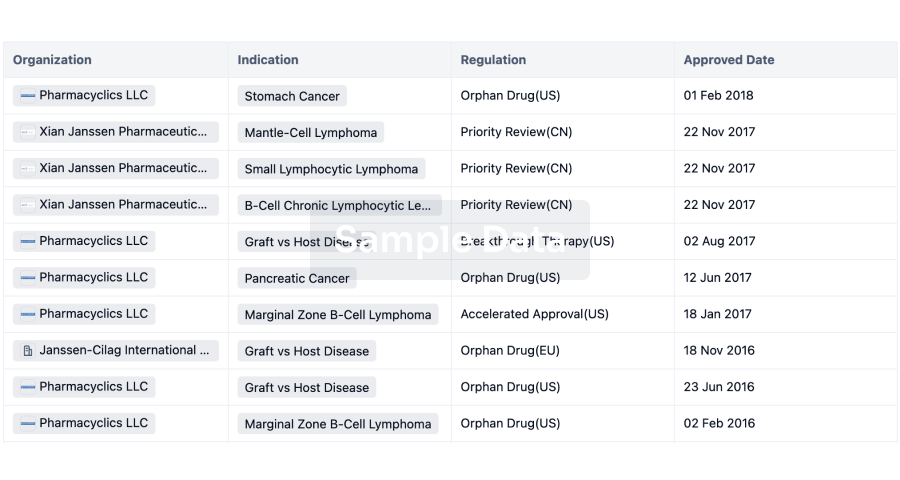
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Leverages most recent intelligence information, enabling fullest potential.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free