/ CompletedNot Applicable 多巴丝肼胶囊在健康受试者中随机、开放、两制剂、单次给药、双周期、双交叉空腹和餐后的生物等效性试验
[Translation] A randomized, open-label, two-dose, single-dose, two-period, double-crossover bioequivalence study of dopamine capsules in healthy volunteers on fasting and fed diets
以上海益生源药业有限公司生产的多巴丝肼胶囊(规格:200 mg/50 mg)为受试制剂,Roche Products Ltd罗氏有限公司(英国)生产的多巴丝肼胶囊(商品名:Madopar,规格200 mg/50 mg)为参比制剂,考察两制剂在空腹及餐后状态下单次给药的药代动力学参数,评价两制剂是否具有生物等效性。
[Translation] Dopamine capsules (specification: 200 mg/50 mg) produced by Shanghai Yishengyuan Pharmaceutical Co., Ltd. were used as the test preparation, and dopamine capsules (trade name: Madopar, specification 200 mg/50 mg) produced by Roche Products Ltd (UK) were used as the reference preparation. The pharmacokinetic parameters of the two preparations after single administration in the fasting and postprandial states were investigated to evaluate whether the two preparations were bioequivalent.
/ CompletedNot Applicable 中国健康受试者空腹和餐后单次口服阿德福韦酯片的随机、开放、两序列、两周期、双交叉设计的生物等效性试验
[Translation] A randomized, open-label, two-sequence, two-period, double-crossover bioequivalence study of a single oral dose of adefovir dipivoxil tablets in Chinese healthy volunteers after fasting or after meals
研究空腹和餐后单次口服阿德福韦酯片受试制剂(优贺丁®,10 mg/片,上海益生源药业有限公司)与阿德福韦酯片参比制剂(Hepsera®,10 mg/片,Gilead Science Inc)后阿德福韦在中国健康受试者体内的药代动力学(Pharmacokinetics,PK)行为,评价空腹和餐后口服两种制剂的生物等效性。 次要目的: 评价中国健康受试者单次口服阿德福韦酯片受试制剂和参比制剂后的安全性。
[Translation] The pharmacokinetic (PK) behavior of adefovir in healthy Chinese subjects was studied after a single oral administration of the test formulation of adefovir dipivoxil tablets (Uhedin®, 10 mg/tablet, Shanghai Yishengyuan Pharmaceutical Co., Ltd.) and the reference formulation of adefovir dipivoxil tablets (Hepsera®, 10 mg/tablet, Gilead Science Inc) on an empty stomach and after a meal, and the bioequivalence of the two formulations after an empty stomach and after a meal was evaluated. Secondary objective: To evaluate the safety of the test formulation and the reference formulation of adefovir dipivoxil tablets in healthy Chinese subjects after a single oral administration.
/ CompletedNot Applicable 多巴丝肼胶囊在健康受试者中随机、开放、两制剂、单次给药、双周期、双交叉空腹状态下的生物等效性预试验
[Translation] A randomized, open-label, two-dose, single-dose, two-period, double-crossover bioequivalence pilot study of dopamine capsules in healthy subjects under fasting conditions
以上海益生源药业有限公司生产的多巴丝肼胶囊(规格:200 mg/50 mg)为受试制剂,Roche Products Ltd罗氏有限公司(英国)生产的多巴丝肼胶囊(商品名:Madopar,规格200 mg/50 mg)为参比制剂,考察两制剂在空腹状态下左旋多巴、苄丝肼的药代动力学参数,验证血中药物浓度分析方法以及采血时间、采样量、时间间隔等设置的合理性。估算变异系数,初步评价两制剂的生物等效性。
[Translation] The test preparation was doubasil capsules (specification: 200 mg/50 mg) produced by Shanghai Yishengyuan Pharmaceutical Co., Ltd., and the reference preparation was doubasil capsules (trade name: Madopar, specification 200 mg/50 mg) produced by Roche Products Ltd (UK). The pharmacokinetic parameters of levodopa and benserazide of the two preparations under fasting state were investigated, and the rationality of the blood drug concentration analysis method and the settings of blood sampling time, sampling volume, time interval, etc. were verified. The coefficient of variation was estimated, and the bioequivalence of the two preparations was preliminarily evaluated.
100 Clinical Results associated with Shanghai Yishengyuan Pharmaceutical Co., Ltd.
0 Patents (Medical) associated with Shanghai Yishengyuan Pharmaceutical Co., Ltd.
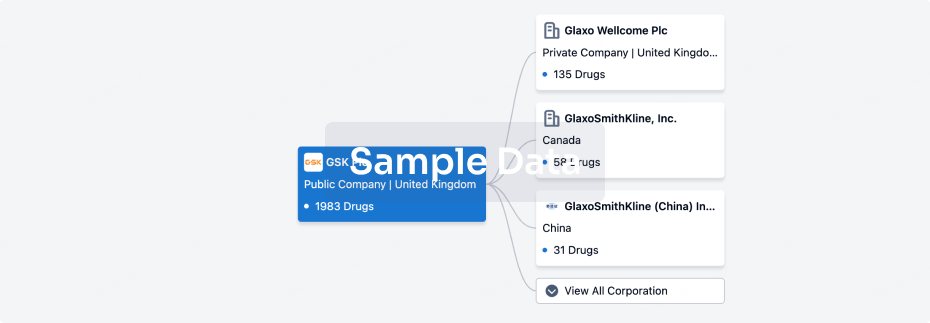
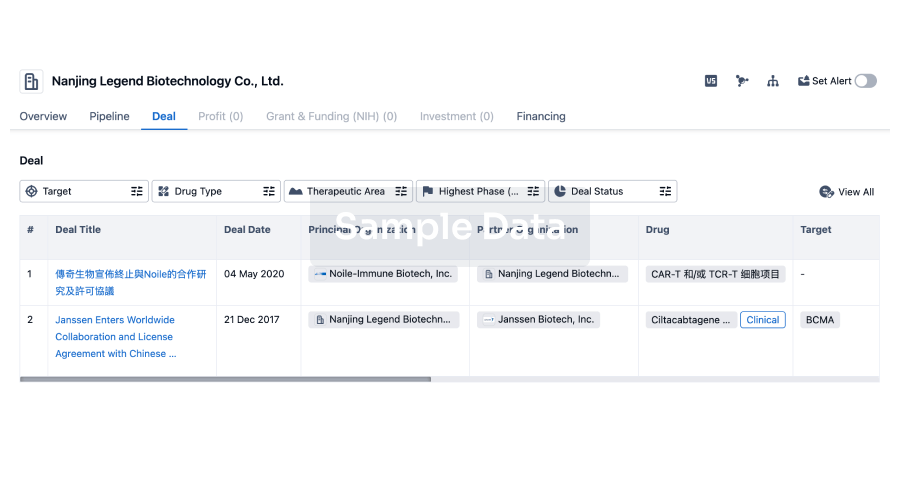
100 Deals associated with Shanghai Yishengyuan Pharmaceutical Co., Ltd.
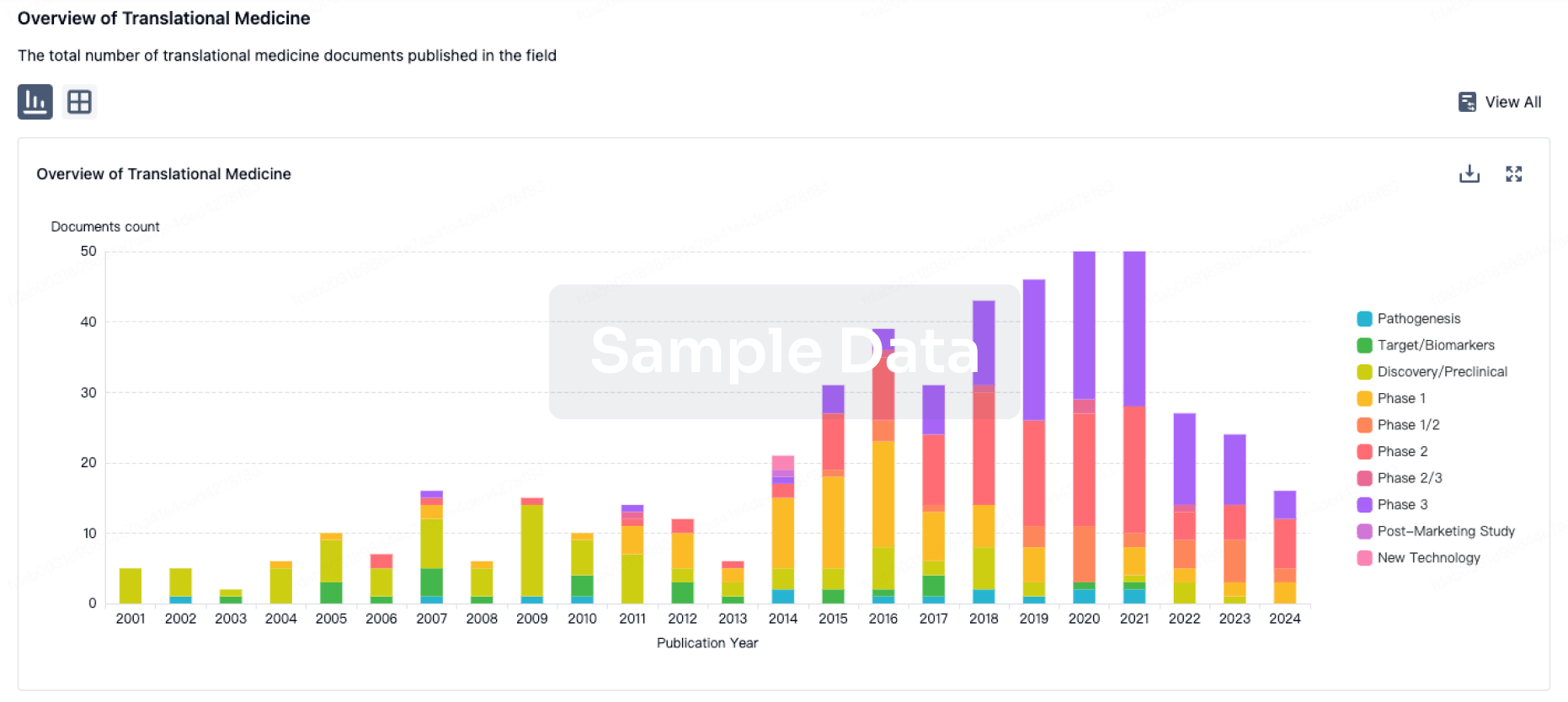
100 Translational Medicine associated with Shanghai Yishengyuan Pharmaceutical Co., Ltd.