2024 AACR Conference: Clinical Progress of ADC Therapeutics
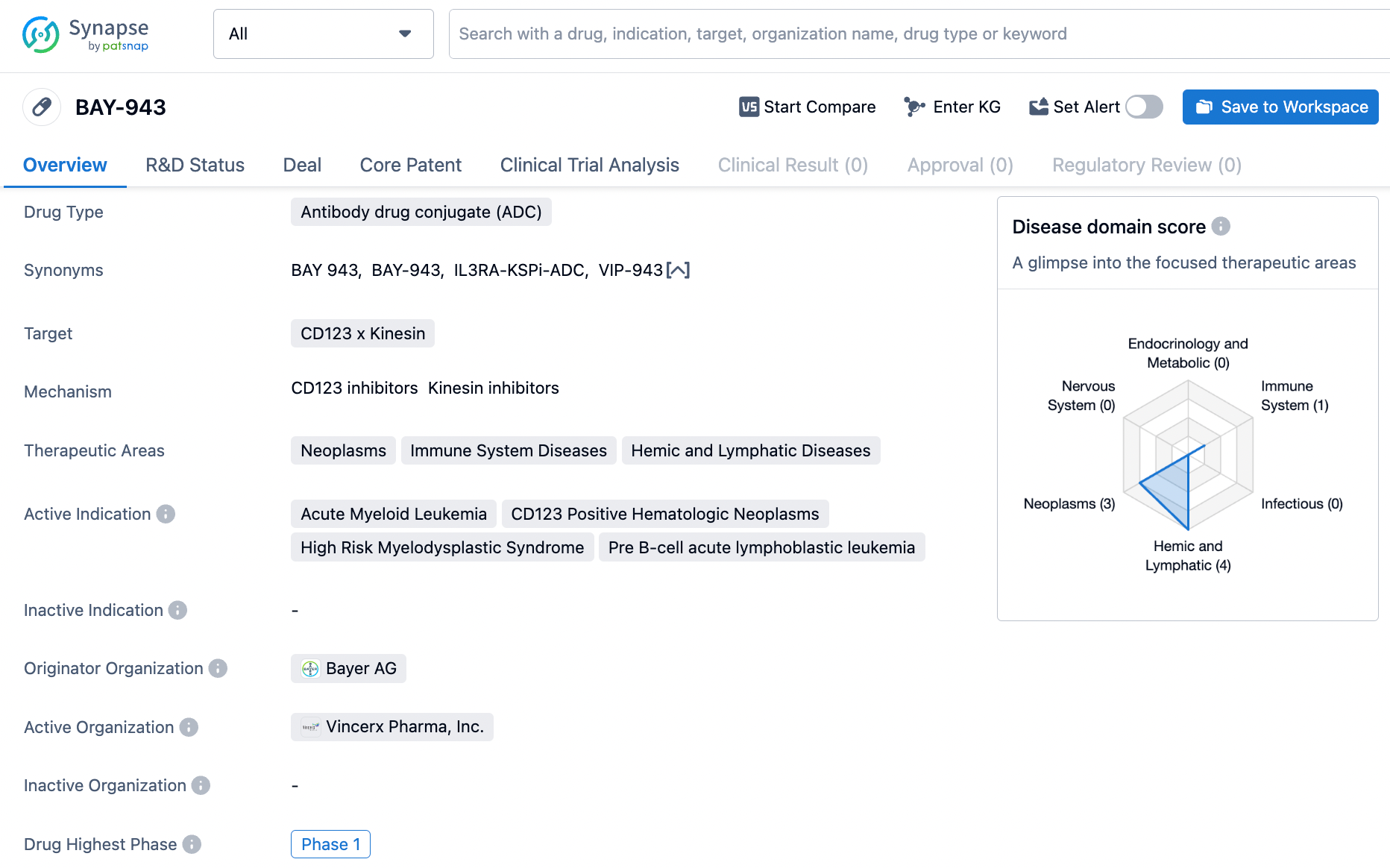
1.Bayer/Vincerx: BAY-943
BAY-943 (VIP943) is the first ADC (Antibody-Drug Conjugate) developed by Vincerx using the company's platform, consisting of an anti-CD123 antibody, a unique linker cleaved by legumain within the cell, and a novel payload of a kinesin spindle protein inhibitor (KSPi). A Phase 1 dose-escalation trial is currently underway to assess the treatment of patients with relapsed/refractory CD123 positive Acute Myeloid Leukemia (AML), B-cell Acute Lymphoblastic Leukemia (B-ALL), or Myelodysplastic Syndromes (MDS) who are unresponsive to standard treatment regimens.
The VNC-943-101 study is an open-label, multi-center, Phase 1 dose-escalation study using VIP943 as a monotherapy in patients with relapsed/refractory AML, B-ALL, and MDS. The primary objective of the trial is to determine the safe dosage of VIP943 for further clinical development. Three patients have been dosed in cohort 1 (0.2 mg/kg), and four patients in cohort 2 (0.4 mg/kg). All seven enrolled patients completed the 28-day DLT (Dose-Limiting Toxicity) assessment period.
Pharmacokinetic (PK) data indicate that unbound free payload is minimal, consistent with the good safety profile observed preclinically and clinically.
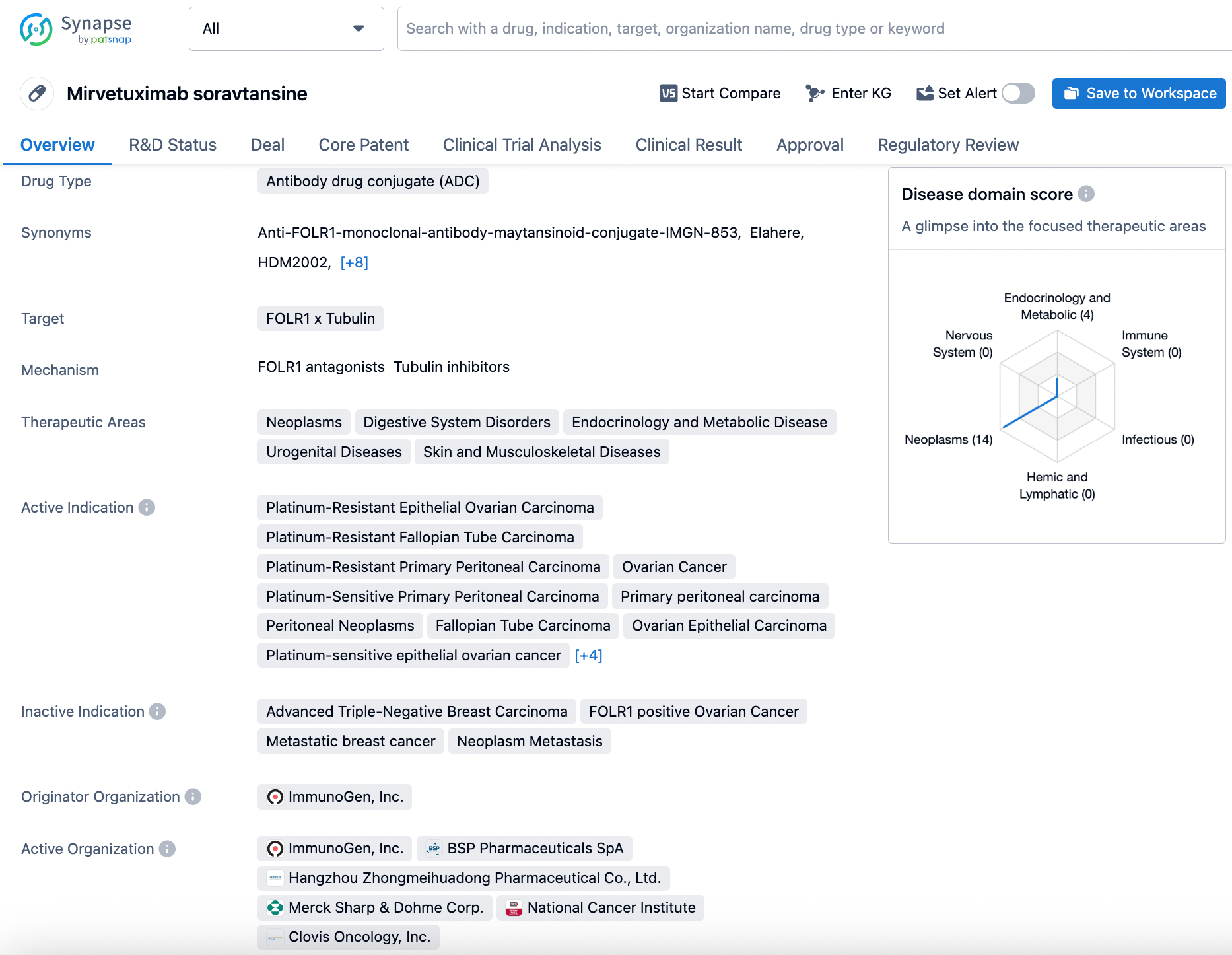
2.AbbVie: Mirvetuximab Soravtansine
Mirvetuximab soravtansine (also known as IMGN853, trade name: ELAHRET™) is the world's first antibody-drug conjugate (ADC) for folate receptor alpha (FRα)-positive ovarian cancer, developed by ImmunoGen, which was acquired by AbbVie. The drug was granted accelerated approval by the FDA in 2022; Huadong Medicine has the exclusive rights to it in Mainland China, Hong Kong, Macau, and Taiwan.
The data disclosed herein pertain to a Phase II trial of mirvetuximab soravtansine (IMGN853) in combination with pembrolizumab for the treatment of patients with microsatellite-stable (MSS) recurrent or persistent endometrial cancer. Previously, the drug demonstrated good tolerability and single-agent activity in a Phase I dose-expansion study in patients with FRα-positive advanced/late-stage disease. The co-primary endpoints of the current study are objective response rate (ORR) measured according to RECIST 1.1 and the frequency of progression-free survival at 6 months (PFS6). Sixteen patients were enrolled in the first phase of the study; if ≥2 objective responses or ≥2 PFS6 responses were observed, an additional 19 patients would be recruited for the second phase. If a total of 4 objective responses or 8 PFS6 events were achieved, the combination would be considered worthy of further investigation.
Results show that as of November 2023, a total of 16 patients had received study treatment, with a median follow-up of 4.7 months (IQR 2.9, 17.3). Among the 16 treated patients, 6 patients (37.5%, 95% CI: 15.2-64.6%) achieved an objective response, including 1 confirmed complete response (CR) and 5 partial responses (PR), with 3 confirmed PRs (18.8%) and 2 unconfirmed PRs (12.5%). Additionally, 31.3% (5/16) of patients had stable disease, with two patients surviving without progression at 6 months.
In terms of safety, the most common treatment-related adverse events (TRAEs) included increased AST (50%), blurred vision (44%), fatigue (44%), and diarrhea (43%); two patients (12.5%) experienced a Grade 3 TRAE. Eye toxicity of any grade was reported in 56.3% of patients.
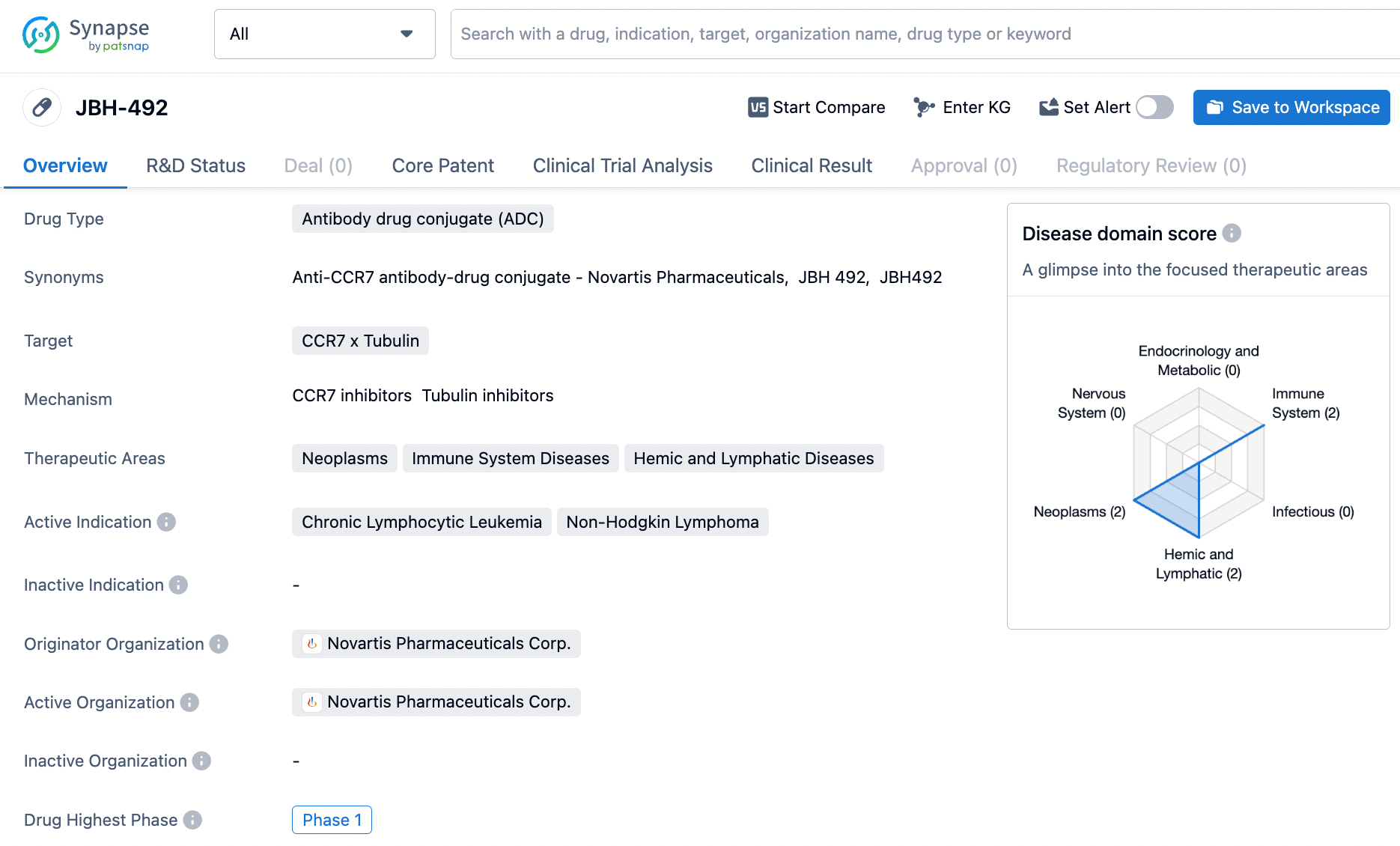
3.Novartis: JBH492
JBH492 is an ADC (Antibody-Drug Conjugate) that targets the CC-Chemokine Receptor 7 (CCR7). It is composed of a humanized anti-CCR7 IgG1 antibody conjugated to the maytansinoid microtubule inhibitor DM4 via a cleavable linker at a specific site. Preclinical studies have shown that this drug possesses effective target-dependent cytotoxic activity, demonstrating potent and durable efficacy in various clinically relevant lymphoma models.
This disclosed study represents an ongoing first-in-human, open-label, phase I/Ib, multi-center trial being conducted in patients with relapsed/refractory (r/r) chronic lymphocytic leukemia (CLL) and non-Hodgkin lymphoma (NHL), which has completed enrollment for the dose-escalation phase. The purpose of dose escalation is to evaluate the safety, tolerability, pharmacokinetics (PK), immunogenicity, and preliminary efficacy of JBH492 and to determine the maximum tolerated dose (MTD) and the recommended dose.
The study indicates that four NHL patients (two with T-cell NHL and two with B-cell NHL) experienced partial remission (PR), and two patients had stable disease, with PR observed at dosages ranging from 2.4 to 3.6 mg/kg every three weeks. Following the data cutoff, one patient with T-cell NHL achieved complete remission (CR) after four treatment cycles. No correlation between CCR7 expression and response could be established (CCR7 expression was observed in all samples from 14 patients).
Regarding safety, 23 patients (92%) experienced at least one adverse event (AE) (Grade ≥ 3, n = 11). Fifteen patients (60%) reported treatment-related AEs (TRAEs) (Grade ≥ 3, n = 3). Of the six patients (24%) who had serious AEs (SAEs) (Grade ≥ 3, n = 3), two (8%) had TRSAEs. The most common AEs (≥ 20%) included anemia (32%), thrombocytopenia (28%), neutropenia, and dry eye syndrome (each 20%). There were two deaths due to underlying diseases.
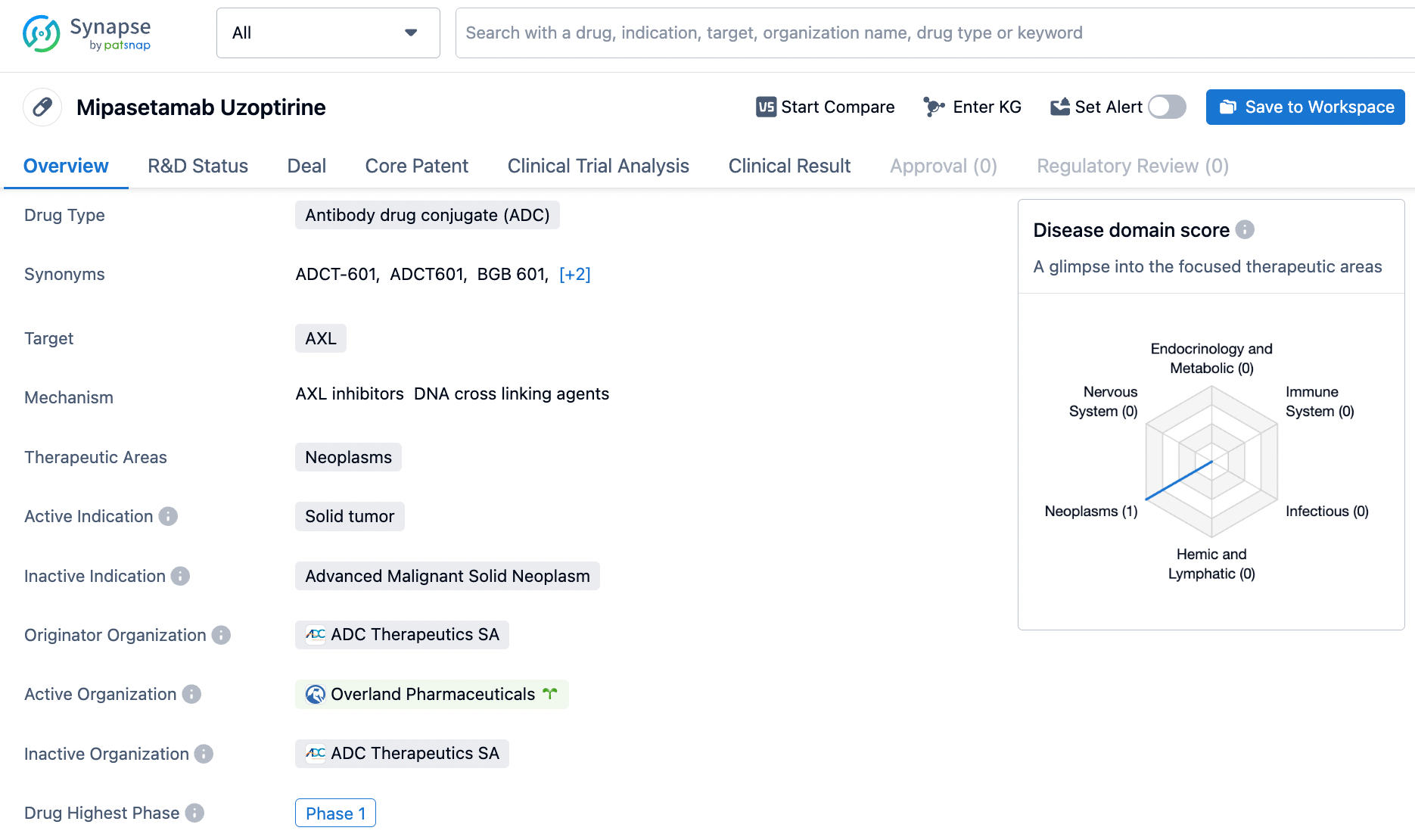
4.ADC Therapeutics: Mipasetamab Uzoptirine
Mipasetamab uzoptirine (ADCT-601) is a targeted Axl Antibody-Drug Conjugate (ADC) that consists of a humanized anti-AXL antibody conjugated to the pyrrolobenzodiazepine (PBD) dimer SG3199 via a cleavable linker. AXL is a cell surface receptor tyrosine kinase that is widely expressed in solid tumors (ST), including sarcomas. ADCT-601 has demonstrated antitumor activity in preclinical mouse models of sarcoma, adrenocortical carcinoma, and pancreatic cancer, and has shown clinical activity in ST patients during its first-in-human trials.
In a Phase 1b, open-label, dose-escalation and dose-expansion study, patients were treated with ADCT-601 in a 3+3 dose-escalation design every three weeks at increasing dose levels. As of December 4, 2023, among 17 evaluable sarcoma patients according to RECISTv1.1, the best overall response was partial response (PR) (2 patients [11.8%]), including confirmed PR; stable disease (SD) (8 patients [47.1%]); and progressive disease (PD) (7 patients [41.2%]). Tumor reduction was observed at the doses of 7.5mg, 11mg, and 13mg. By week 12, 6/17 patients (35.3%) had not exhibited progression of disease (PD).
In terms of safety, the most common treatment-emergent adverse events (TEAEs) of all grades and relationships (≥20%) were hand-foot syndrome (7 patients [38.9%]); anemia (6 patients [33.3%]); maculopapular rash (5 patients [27.8%]); cheilitis and constipation (4 patients each [22.2%]). TEAEs of grade ≥3 occurred in 9 patients (50%). The most common grade ≥3 TEAEs (≥10%) were increased GGT (2 patients [11.1%]). Two dose-limiting toxicities were observed: grade 2 and grade 3 cheilitis at doses of 15mg and 13mg, respectively. Skin reactions of all grades were more pronounced at higher doses. The maximum tolerated dose (MTD) has not yet been determined.
5.Roche: Trastuzumab Emtansine
Trastuzumab emtansine is a HER2-targeted ADC that has been approved for the treatment of metastatic breast cancer with HER2 overexpression. HER2 point mutations are present in approximately 1.4% of solid tumors, but the prevalence varies among different tumor types.
The TAPISTRY study disclosed here is a Phase II, global, open-label, multi-cohort trial designed to assess the efficacy and safety of several therapies in patients with advanced/metastatic solid tumors. The primary endpoint is the Objective Response Rate (ORR) as assessed by an Independent Review Committee (IRC), with key secondary endpoints including the ORR assessed by the researchers; duration of response; progression-free survival; overall survival rate; and drug safety.
As of July 16, 2023, 35 patients with 10 different tumor types were evaluable for efficacy, with Non-Small Cell Lung Cancer (NSCLC) being the most common. After a median follow-up of 7 months, the ORR assessed by IRC for patients evaluable for efficacy was 14.3%; responses were observed in three tumor types: NSCLC (n/N=1/11), breast cancer (n/N=3/5), and endometrial carcinoma (n/N=1/1). The most common adverse events were decreased appetite, fatigue, and nausea (25.7% each). No new safety signals were found.
6.Heidelberg: HDP-101
HDP-101 is a BCMA-targeted ADC (antibody-drug conjugate) developed by Heidelberg, consisting of a BCMA antibody, VA-PABC linker, and an Amanitin derivative HDP 30.2115. Due to its high toxicity, the drug-to-antibody ratio (DAR) is set at 2, and a site-specific conjugation method is used to ensure homogeneity and reduce potential toxicity. As an immunoactivating agent, HDP-101 can induce immunogenic cell death (ICD) and shows synergistic effects with immune checkpoint inhibitors, offering possibilities for combination therapy in a clinical setting.
The data disclosed here pertains to a first-in-human, open-label, non-randomized, multi-center, phase 1/2a trial aimed at determining the maximum tolerated dose and establishing a phase 2 trial recommended dose in patients with multiple myeloma. As of November 2023, 18 patients (7 females and 11 males) have been enrolled across 5 sequential dose cohorts with doses of 20, 30, 60, 80, and 100 µg/kg.
Preliminary data indicates that the pharmacokinetics of HDP-101 are in line with expectations, and the exposure to HDP-101 is dose-proportional. Patients in cohort 3 exhibited stable disease (SD) after 15 cycles. In cohort 5, there are 4 ongoing patients who have shown promising results after 3-4 cycles: 2 patients achieved partial response (PR), and 2 patients reached SD.
In terms of safety, the drug was well tolerated with no dose-limiting toxicities (DLT), including no signs of hepatorenal toxicity, infusion reactions, or ocular diseases. Of the 18 patients across the 5 treatment groups, 17 were evaluable for DLT assessment.
7.Daiichi Sankyo/AstraZeneca: Trastuzumab Deruxtecan
Trastuzumab deruxtecan (T-DXd), also known as fam-trastuzumab deruxtecan-nxki, is an antibody-drug conjugate (ADC) that targets HER2, composed of a humanized anti-HER2 antibody, an enzymatically cleavable peptide linker, and a topoisomerase I inhibitor. The disclosed phase 2 DESTINY-Lung05 (DL-05) trial was conducted to evaluate its efficacy in Chinese patients with previously treated HER2-mutant non-small cell lung cancer (NSCLC). This open-label, single-arm, phase 2 trial primarily aimed to assess the objective response rate (ORR) confirmed by an Independent Central Review (ICR); secondary endpoints included ORR confirmed by investigator's assessment (INV), duration of response, disease control rate, progression-free survival, and safety evaluated by both ICR and INV.
The results, as of September 23, 2023, demonstrated that the median exposure to T-DXd was 7.9 (0.7-13.5) months. A total of 71 patients experienced drug-related adverse events (AEs), with 51.4% being grade (G) ≥3. The most common G≥3 AEs by grouped terms included neutropenia (26.4%), thrombocytopenia (18.1%), and leukopenia (11.1%). Two patients (2.8%) experienced drug-related AEs leading to discontinuation of treatment. Seventeen patients (23.6%) experienced serious AEs with no G5 determined by INV. Central adjudication identified drug-related interstitial lung disease (ILD)/pneumonitis in 7 patients (9.7%; n=6 G2; n=1 G5). This suggests that the safety profile in the Chinese patient population is consistent with the known safety data.