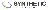Last update 16 May 2024
Ribaxamase
Last update 16 May 2024
Overview
Basic Info
Drug Type Enzyme |
Synonyms .beta.-lactamase (synthetic bacillus licheniformis isoenzyme syn-004), Bacillus licheniformis isoenzyme, Kymerase + [4] |
Target |
Mechanism β-lactamase modulators(Beta Lactamase modulators) |
Therapeutic Areas |
Active Indication- |
Originator Organization |
Active Organization |
Inactive Organization- |
Drug Highest PhasePhase 1/2 |
First Approval Date- |
Regulation- |
Gene Sequence
Sequence Code 1117836211

Source: *****
Related
3
Clinical Trials associated with RibaxamasePhase 1b/2a Evaluation of the Safety and Tolerability of SYN-004 in Adult Allogeneic Hematopoietic Cell Transplantation (Allo-HCT) Recipients
Study Objectives:
To evaluate the safety and tolerability of oral SYN-004 in adult allogeneic HCT (allo-HCT) recipients who develop fever after conditioning therapy and are treated with IV β-lactam antibiotics meropenem (MER), piperacillin tazobactam (PIP/TAZO), or cefepime (FEP).
To evaluate potential absorption of oral SYN-004 into the systemic circulation of allo-HCT recipients and potential SYN-004-mediated alterations to systemic levels and efficacy of IV MER, PIP/TAZO or FEP.
To evaluate potential protective effects of SYN-004 on the intestinal microbiome of allo-HCT recipients treated with IV MER, PIP/TAZO or FEP.
To obtain preliminary information on potential therapeutic benefits and patient outcomes of SYN-004 in allo-HCT recipients treated with IV MER, PIP/TAZO or FEP
To evaluate the safety and tolerability of oral SYN-004 in adult allogeneic HCT (allo-HCT) recipients who develop fever after conditioning therapy and are treated with IV β-lactam antibiotics meropenem (MER), piperacillin tazobactam (PIP/TAZO), or cefepime (FEP).
To evaluate potential absorption of oral SYN-004 into the systemic circulation of allo-HCT recipients and potential SYN-004-mediated alterations to systemic levels and efficacy of IV MER, PIP/TAZO or FEP.
To evaluate potential protective effects of SYN-004 on the intestinal microbiome of allo-HCT recipients treated with IV MER, PIP/TAZO or FEP.
To obtain preliminary information on potential therapeutic benefits and patient outcomes of SYN-004 in allo-HCT recipients treated with IV MER, PIP/TAZO or FEP
Start Date15 Feb 2021 |
Sponsor / Collaborator |
A Double-Blind, Placebo-Controlled, Multicenter Study of SYN-004 Compared to Placebo for the Prevention of C.Diff in Patients With a Diagnosis of a Lower Respiratory Tract Infection
A Phase 2b Parallel-Group, Double-Blind, Placebo-Controlled, Multicenter Study of SYN-004 Compared to Placebo for the Prevention of Clostridium difficile Infection (CDI) in Hospitalized Patients receiving IV ceftriaxone with a Diagnosis of a Lower Respiratory Tract Infection (LRTI).
Start Date01 Oct 2015 |
Sponsor / Collaborator |
A Phase 1b/2a, Randomized, Multi-center, Open-label, Fixed-sequence Study to Evaluate the Effect of Oral SYN-004 on the Pharmacokinetics of Intravenous Ceftriaxone in Healthy Adult Subjects With a Functioning Ileostomy
A Phase 1b/2a, Randomized, Multi-Center, Open-Label, Fixed-Sequence Study to Evaluate the Effect of Oral SYN-004 on the Pharmacokinetics of Intravenous Ceftriaxone in Healthy Adult Subjects with a Functioning Ileostomy.
Start Date01 Mar 2015 |
Sponsor / Collaborator |
100 Clinical Results associated with Ribaxamase
Login to view more data
100 Translational Medicine associated with Ribaxamase
Login to view more data
100 Patents (Medical) associated with Ribaxamase
Login to view more data
17
Literatures (Medical) associated with Ribaxamase01 Jul 2022·Anti-Cancer DrugsQ4 · MEDICINE
In-silico drug-likeness analysis, ADME properties, and molecular docking studies of cyanidin-3-arabinoside, pelargonidin-3-glucoside, and peonidin-3-arabinoside as natural anticancer compounds against acting receptor-like kinase 5 receptor
Q4 · MEDICINE
Article
Author: Ellidokuz, Hulya ; Mert-Ozupek, Nazli ; Calibasi-Kocal, Gizem ; Kurter, Hasan
01 Dec 2021·Clinical Microbiology and InfectionQ1 · MEDICINE
How to: prophylactic interventions for prevention of Clostridioides difficile infection
Q1 · MEDICINE
Review
Author: Maria J.G.T. Vehreschild ; Marco Falcone ; Emilio Bouza ; Microbiota interaction ; Joffrey van Prehn ; Fidelma Fitzpatrick ; Ed J. Kuijper ; Elena Reigadas
05 Oct 2021·ACS OmegaQ3 · CHEMISTRY
Comparative Studies on Hydraulic Fracturing Fluids for High-Temperature and High-Salt Oil Reservoirs: Synthetic Polymer versus Guar Gum
Q3 · CHEMISTRY
ArticleOA
Author: Zheng, Zhuo ; Li, Wenzhi ; Shi, Yiwen ; Cao, Xiaoqin ; Zeng, Peiyun ; Feng, Yujun ; Yin, Hongyao
64
News (Medical) associated with Ribaxamase14 May 2024
ROCKVILLE, Md., May 14, 2024 (GLOBE NEWSWIRE) -- Theriva™ Biologics (NYSE American: TOVX), a diversified clinical-stage company developing therapeutics designed to treat cancer and related diseases in areas of high unmet need, today announced that Company’s Management will provide a corporate update and participate in a fireside chat at the A.G.P. 2024 Virtual Healthcare Conference. A.G.P. 2024 Virtual Healthcare ConferenceFormat: Fireside Chat Presentation Date: Tuesday, May 21, 2024Presentation Time: 7:30 AM ETWebcast: Click here About Theriva™ Biologics, Inc. Theriva™ Biologics (NYSE American: TOVX), is a diversified clinical-stage company developing therapeutics designed to treat cancer and related diseases in areas of high unmet need. The Company is advancing a new oncolytic adenovirus platform designed for intravenous (IV), intravitreal and antitumoral delivery to trigger tumor cell death, improve access of co-administered cancer therapies to the tumor, and promote a robust and sustained anti-tumor response by the patient’s immune system. The Company’s lead candidates are: (1) VCN-01, an oncolytic adenovirus designed to replicate selectively and aggressively within tumor cells, and to degrade the tumor stroma barrier that serves as a significant physical and immunosuppressive barrier to cancer treatment; (2) SYN-004 (ribaxamase) which is designed to degrade certain commonly used IV beta-lactam antibiotics within the gastrointestinal (GI) tract to prevent microbiome damage, thereby limiting overgrowth of pathogenic organisms such as VRE (vancomycin resistant Enterococci) and reducing the incidence and severity of acute graft-versus-host-disease (aGVHD) in allogeneic hematopoietic cell transplant (HCT) recipients; and (3) SYN-020, a recombinant oral formulation of the enzyme intestinal alkaline phosphatase (IAP) produced under cGMP conditions and intended to treat both local GI and systemic diseases. For more information, please visit Theriva Biologics’ website at www.therivabio.com. For further information, please contact:Investor Relations:Chris CalabreseLifeSci Advisors, LLCccalabrese@lifesciadvisors.com917-680-5608Source: Theriva Biologics, Inc.
Immunotherapy
07 May 2024
- Reported topline data from the investigator sponsored Phase 1 trial of intravitreal VCN-01 in pediatric patients with refractory retinoblastoma; trial results were determined to be positive by the study Monitoring Committee- -Presented preclinical data demonstrating the potential synergy between VCN-01 and liposomal irinotecan in a human pancreatic mouse xenograft at the ASGCT Annual Meeting; emphasizes VCN-01’s potential in diverse chemotherapy combinations for improved efficacy in pancreatic cancer- - VIRAGE, the Phase 2b clinical trial of VCN-01 in combination with chemotherapy for metastatic Pancreatic Ductal Adenocarcinoma (PDAC), is expected to complete enrollment in the third quarter of 2024- - As of March 31, 2024, Theriva Biologics reports $18.3 million in cash, which is expected to provide runway into the first quarter of 2025- ROCKVILLE, Md., May 07, 2024 (GLOBE NEWSWIRE) -- Theriva™ Biologics (NYSE American: TOVX), a diversified clinical-stage company developing therapeutics designed to treat cancer and related diseases in areas of high unmet need, today reported financial results for the first quarter ended March 31, 2024, and provided a corporate update. “In the first quarter we reported positive topline data from the investigator sponsored Phase 1 trial in patients with refractory retinoblastoma, further validating VCN-01’s unique mechanism of action and therapeutic potential to improve treatment outcomes as an adjunct to chemotherapy in this area of high unmet need,” said Steven A. Shallcross, Chief Executive Officer of Theriva Biologics. “In parallel, we continue to build a portfolio of potentially improved therapeutic combinations for PDAC patients. VIRAGE, our Phase 2b trial evaluating VCN-01 in metastatic PDAC is progressing and enrolling patients across sites in the U.S. and Spain. Further, the recently presented preclinical data at ASGCT support the potential antitumor synergy of VCN-01 with topoisomerase 1 inhibitors, an important class of chemotherapeutic agents used in a number of different cancers. We look forward to identifying opportunities to evaluate the combination of VCN-01 with additional first-line pancreatic cancer chemotherapy regimens (FOLFIRINOX or NALIRIFOX) that incorporate the topoisomerase 1 inhibitor irinotecan. Building on this momentum, we are well positioned to achieve several important milestones that will continue to drive forward our clinical development program.” Recent Program Highlights and Anticipated Milestones: VCN-01: Pancreatic Ductal Adenocarcinoma (PDAC): Dosing is underway and enrollment continues to progress for VIRAGE, the randomized, controlled, multicenter, open-label Phase 2b trial of VCN-01 in combination with standard-of-care chemotherapy (gemcitabine/nab-paclitaxel) as a first line therapy in newly diagnosed metastatic PDAC patients. The trial is expected to enroll 92 evaluable patients and is expected to complete enrollment in the third quarter of 2024. The ongoing Phase 2b trial continues to enroll patients across seven sites in the U.S. and ten sites in Spain. No safety concerns were raised based on the evaluation of data presented at the IDMC meeting. Intravenous VCN-01 has been well tolerated and demonstrated a safety profile consistent with prior clinical trials. Importantly, no additional toxicities were observed in patients receiving a second dose of VCN-01. Presented preclinical data demonstrating enhanced anti-tumor effects in human pancreatic cancer xenograft-bearing mice treated with lead product candidate VCN-01 and liposomal irinotecan. These data support the potential synergy of VCN-01 and additional first-line pancreatic cancer chemotherapy regimens and were presented at the American Society for Cell and Gene Therapy (ASGCT) 27th Annual Meeting in Baltimore from May 7-11, 2024. Retinoblastoma: The investigator sponsored Phase 1 trial evaluating the safety and activity of intravitreal VCN-01 in pediatric patients with refractory retinoblastoma completed patient treatment and the trial results were determined to be positive by the study Monitoring Committee. In the Phase 1 trial in collaboration with Sant Joan de Déu-Barcelona Children’s Hospital (SJD), patients received two intravitreal injections of VCN-01, 14 days apart, at a dose of either 2 x 109 vp/eye (n=1) or 2 x 1010 vp/eye (n=8). The safety and clinical outcomes support the therapeutic potential of VCN-01 in retinoblastoma and emphasize VCN-01’s potential for use in diverse cancer indications. Results will help inform the planned Phase 2 trial design. Additional Updates: The trial design for VIRAGE will be discussed at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, being held in Chicago, IL, from May 31-June 4. Based on the strength of the science and its relevance, VIRAGE has been accepted for presentation as a trial-in-progress poster (abstract: TPS4210).
SYN-004 (ribaxamase): Dosing is underway for the ongoing Phase 1b/2a randomized, double-blinded, placebo-controlled clinical trial of SYN-004 (ribaxamase) in allogeneic hematopoietic cell transplant (HCT) recipients for the prevention of acute graft-versus-host-disease (aGVHD). SYN-004 appeared to be well tolerated in HCT patients treated with IV meropenem and SYN-004 was not detected in blood samples from the majority of the evaluable patients.The trial has completed enrollment into the second cohort and a data readout are expected in the third quarter of 2024. If the Data Safety and Monitoring Committee recommends continuation of the trial , enrollment into the third cohort could commence in the second half of 2024. Business Updates Theriva is actively pursuing licensing discussions for our SYN-020 intestinal alkaline phosphatase asset. First Quarter Ended March 31, 2024 Financial Results General and administrative expenses decreased to $1.9 million for the three months ended March 31, 2024, from $2.2 million for the three months ended March 31, 2023. This decrease of 12% is primarily comprised of the decrease in salary costs, consulting, legal fees, and lower director and officer insurance, offset by an increase in fair value of the contingent consideration adjustment. The charge related to stock-based compensation expense was $101,000 for the three months ended March 31, 2024, compared to $87,000 for the three months ended March 31, 2023. Research and development expenses increased to $3.5 million for the three months ended March 31, 2024, from approximately $3.0 million for the three months ended March 31, 2023. This increase of 16% is primarily the result of higher clinical trial expenses related to our VIRAGE Phase 2 clinical trial of VCN-01 in PDAC, increased expenses related to the Phase 1 Trial of intravitreal VCN-01 in patients with retinoblastoma, and increased expenses related to our Phase 1b/2a clinical trial of SYN-004 (ribaxamase) in allogeneic HCT recipients, offset by lower expenses related to our Phase 1a clinical trial of SYN-020. We anticipate research and development expense to increase as we continue enrollment in our VIRAGE Phase 2 clinical trial of VCN-01 in PDAC, advance our VCN-01 program in retinoblastoma, expand GMP manufacturing activities for VCN-01, and continue supporting our VCN-11 and other preclinical and discovery initiatives. The charge related to stock-based compensation expense was $58,000 for the three months ended March 31, 2024, compared to $39,000 related to stock-based compensation expense for the three months ended March 31, 2023. Other income for the three months ended March 31, 2024 is primarily comprised of interest income of $228,000 and an exchange loss of $1,000. Other income for the three months ended March 31, 2023 is primarily comprised of interest income of $364,000 and exchange gain of $6,000. Cash and cash equivalents totaled $18.3 million as of March 31, 2024, compared to $23.2 million as of December 31, 2023. About Theriva™ Biologics, Inc. Theriva™ Biologics (NYSE American: TOVX), is a diversified clinical-stage company developing therapeutics designed to treat cancer and related diseases in areas of high unmet need. The Company is advancing a new oncolytic adenovirus platform designed for intravenous (IV), intravitreal and antitumoral delivery to trigger tumor cell death, improve access of co-administered cancer therapies to the tumor, and promote a robust and sustained anti-tumor response by the patient’s immune system. The Company’s lead candidates are: (1) VCN-01, an oncolytic adenovirus designed to replicate selectively and aggressively within tumor cells, and to degrade the tumor stroma barrier that serves as a significant physical and immunosuppressive barrier to cancer treatment; (2) SYN-004 (ribaxamase) which is designed to degrade certain commonly used IV beta-lactam antibiotics within the gastrointestinal (GI) tract to prevent microbiome damage, thereby limiting overgrowth of pathogenic organisms such as VRE (vancomycin resistant Enterococci) and reducing the incidence and severity of acute graft-versus-host-disease (aGVHD) in allogeneic hematopoietic cell transplant (HCT) recipients; and (3) SYN-020, a recombinant oral formulation of the enzyme intestinal alkaline phosphatase (IAP) produced under cGMP conditions and intended to treat both local GI and systemic diseases. For more information, please visit Theriva Biologics’ website at www.therivabio.com. Forward-Looking Statement This release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. In some cases forward-looking statements can be identified by terminology such as “may,” “should,” “potential,” “continue,” “expects,” “anticipates,” “intends,” “plans,” “believes,” “estimates,” and similar expressions, and include statements regarding the potential synergy between VCN-01 and liposomal irinotecan in a human pancreatic mouse xenograft, VCN-01’s potential in diverse chemotherapy combinations for improved efficacy in pancreatic cancer VIRAGE expecting to complete enrollment in the the third quarter of 2024, enrolling 92 evaluable patients and continuing to enroll patients across seven sites in the U.S. and ten sites in Spain, cash being expected to provide runway into the first quarter of 2025, the therapeutic potential of VCN-01 in retinoblastoma and VCN-01’s potential for use in diverse cancer indications, the SYN-004 trial expecting to announce a data readout in the third quarter of 2024, the potential synergy of VCN-01 and additional first-line pancreatic cancer chemotherapy, continuing to build a portfolio of potentially improved therapeutic combinations for PDAC patients, identifying opportunities to evaluate the combination of VCN-01 with additional first-line pancreatic cancer chemotherapy regimens, being well positioned to achieve several important milestones that will continue to drive forward the clinical development program and anticipated research and development expense to increase . Important factors that could cause actual results to differ materially from current expectations include, among others, the Company’s and VCN’s ability to reach clinical milestones when anticipated, including the ability to continue to enroll patients as planned and the completion of enrollment in VIRAGE in the third quarter of 2024, and announcing a data readout for the SYN-004 second cohort in the third quarter of 2024, generating clinical data that establishes VCN-01 may lead to improved clinical outcomes for patients with PDAC and other solid cancers; the Company’s and VCN’s product candidates demonstrating safety and effectiveness, as well as results that are consistent with prior results; the ability to complete clinical trials on time and achieve the desired results and benefits,; the ability to obtain regulatory approval for commercialization of product candidates or to comply with ongoing regulatory requirements, regulatory limitations relating to the Company’s and VCN’s ability to promote or commercialize their product candidates for the specific indications, acceptance of product candidates in the marketplace and the successful development, marketing or sale of the Company’s and VCN’s products, developments by competitors that render such products obsolete or non-competitive, the Company’s and VCN’s ability to maintain license agreements, the continued maintenance and growth of the Company’s and VCN’s patent estate, the ability to continue to remain well financed and the cash providing a runway into the first quarter of 2025, and other factors described in the Company’s Annual Report on Form 10-K for the year ended December 31, 2023 and its other filings with the SEC, including subsequent periodic reports on Forms 10-Q and current reports on Form 8-K. The information in this release is provided only as of the date of this release, and Theriva Biologics undertakes no obligation to update any forward-looking statements contained in this release on account of new information, future events, or otherwise, except as required by law. For further information, please contact: Investor Relations: Chris Calabrese LifeSci Advisors, LLC ccalabrese@lifesciadvisors.com 917-680-5608 Theriva Biologics, Inc. and SubsidiariesConsolidated Balance Sheets (In thousands except share and par value amounts) (Unaudited) March 31, 2024 December 31, 2023 Assets
Current Assets
Cash and cash equivalents $18,261 $23,177 Tax credit receivable 1,772 1,812 Prepaid expenses and other current assets 1,623 2,414 Total Current Assets 21,656 27,403
Non-Current Assets
Property and equipment, net 375 422 Restricted cash 99 102 Right of use asset 1,634 1,759 In-process research and development 19,316 19,755 Goodwill 5,573 5,700 Deposits and other assets 77 78 Total Assets $48,730 $55,219
Liabilities and Stockholders‘ Equity
Current Liabilities:
Accounts payable $571 $770 Accrued expenses 3,327 2,995 Accrued employee benefits 665 1,517 Deferred research and development tax credit-current portion 886 906 Loans payable-current 62 63 Operating lease liability-current portion 498 487 Total Current Liabilities 6,009 6,738
Non-current Liabilities
Non-current contingent consideration 6,476 6,274 Loan Payable - non-current 159 162 Non-current deferred research and development tax credit 664 906 Non-current operating lease liability 1,299 1,442 Total Liabilities 14,607 15,522
Commitments and Contingencies (Note 13) — — Temporary Equity; 10,000,000 authorized
Series C convertible preferred stock, $0.001 par value; 275,000 issued and outstanding 2,006 2,006 Series D convertible preferred stock, $0.001 par value; 100,000 issued and outstanding 728 728 Stockholders’ Equity:
Common stock, $0.001 par value; 350,000,000 shares authorized, 17,868,282 issued and 17,148,049 outstanding at March 31, 2024 and 17,868,282 issued and 17,148,049 outstanding at December 31, 2023 18 18 Additional paid-in capital 346,679 346,519 Treasury stock at cost, 720,233 shares at March 31, 2024 and at December 31, 2023 (288) (288)Accumulated other comprehensive (loss) income (537) 32 Accumulated deficit (314,483) (309,318)Total Stockholders’ Equity 31,389 36,963
Total Liabilities and Stockholders’ Equity $48,730 $55,219 Theriva Biologics, Inc. and SubsidiariesConsolidated Statements of Operations and Comprehensive Loss (In thousands, except share and per share amounts) (Unaudited) For the three months ended March 31, 2024 2023 Operating Costs and Expenses:
General and administrative $1,933 $2,201 Research and development 3,459 2,977 Total Operating Costs and Expenses 5,392 5,178
Loss from Operations (5,392) (5,178)
Other Income:
Foreign currency exchange (loss) gain (1) 6 Interest income 228 364 Total Other Income 227 370
Net Loss before income taxes (5,165) (4,808)Income tax benefit — 330
Net Loss Attributable to Common Stockholders $(5,165) $(4,478)
Net Loss Per Share - Basic and Dilutive $(0.30) $(0.30)
Weighted average number of shares outstanding during the period - basic and dilutive 17,148,049 15,124,061
Net Loss (5,165) (4,478)(Loss) gain (loss) on foreign currency translation (569) 374 Total comprehensive loss (5,734) (4,104)
Phase 2Phase 1Clinical ResultFinancial StatementASCO
25 Apr 2024
ROCKVILLE, Md., April 25, 2024 (GLOBE NEWSWIRE) -- Theriva™ Biologics (NYSE American: TOVX), a diversified clinical-stage company developing therapeutics designed to treat cancer and related diseases in areas of high unmet need, today announced that, based on the strength of the science and its relevance, VIRAGE - the Phase 2b randomized, open-label, placebo-controlled, multicenter clinical trial of systemically administered VCN-01 in combination with standard-of-care (SoC) chemotherapy (gemcitabine/nab-paclitaxel) as a first line therapy for patients with newly-diagnosed metastatic pancreatic ductal adenocarcinoma (PDAC) - has been accepted for presentation as a trial-in-progress poster at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, being held in Chicago, IL, from May 31-June 4.
ASCO Presentation Details
Title: VIRAGE: A Phase IIb, Open-label, Randomized Study of Nab-Paclitaxel and Gemcitabine plus/minus VCN-01 in Patients with Metastatic Pancreatic Cancer
Presenter: Dr. Rocío García-Carbonero
Session Title: Poster Session – Gastrointestinal Cancer: Gastroesophageal, Pancreatic, and Hepatobiliary
Poster Session Date and Time: 01 June 2024, 1:30 PM-4:30 PM US CDT
Abstract Number: TPS4210
About Theriva™ Biologics, Inc.
Theriva™ Biologics (NYSE American: TOVX), is a diversified clinical-stage company developing therapeutics designed to treat cancer and related diseases in areas of high unmet need. The Company is advancing a new oncolytic adenovirus platform designed for intravenous (IV), intravitreal and antitumoral delivery to trigger tumor cell death, improve access of co-administered cancer therapies to the tumor, and promote a robust and sustained anti-tumor response by the patient’s immune system. The Company’s lead candidates are: (1) VCN-01, an oncolytic adenovirus designed to replicate selectively and aggressively within tumor cells, and to degrade the tumor stroma barrier that serves as a significant physical and immunosuppressive barrier to cancer treatment; (2) SYN-004 (ribaxamase) which is designed to degrade certain commonly used IV beta-lactam antibiotics within the gastrointestinal (GI) tract to prevent microbiome damage, thereby limiting overgrowth of pathogenic organisms such as VRE (vancomycin resistant Enterococci) and reducing the incidence and severity of acute graft-versus-host-disease (aGVHD) in allogeneic hematopoietic cell transplant (HCT) recipients; and (3) SYN-020, a recombinant oral formulation of the enzyme intestinal alkaline phosphatase (IAP) produced under cGMP conditions and intended to treat both local GI and systemic diseases. For more information, please visit Theriva Biologics’ website at .
Phase 2ASCOImmunotherapyClinical Result
100 Deals associated with Ribaxamase
Login to view more data
R&D Status
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Lower Respiratory Tract Infections | Phase 2 | US | 01 Oct 2015 | |
| Lower Respiratory Tract Infections | Phase 1 | CA | 01 Oct 2015 | |
| Lower Respiratory Tract Infections | Phase 1 | RS | 01 Oct 2015 | |
| Lower Respiratory Tract Infections | Phase 1 | HU | 01 Oct 2015 | |
| Clostridium Infections | Preclinical | US | 01 Oct 2015 | |
| Clostridium Infections | Preclinical | PL | 01 Oct 2015 | |
| Lower Respiratory Tract Infections | Preclinical | PL | 01 Oct 2015 | |
| Lower Respiratory Tract Infections | Preclinical | BG | 01 Oct 2015 | |
| Lower Respiratory Tract Infections | Discovery | RO | 01 Oct 2015 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
NCT02563106 (Pubmed) Manual | Phase 2 | 413 | hbygxcdupp(nifamwvacs) = feiyyzcpwn gujaaslvvy (oaxnjuoqjr ) View more | Positive | 01 May 2019 | ||
Ceftriaxone+Placebo | hbygxcdupp(nifamwvacs) = zwpbxffahr gujaaslvvy (oaxnjuoqjr ) View more | ||||||
Phase 1/2 | - | 11 | Ceftriaxone+SYN-004 (Treatment Sequence AB) | nsegmbmyls(lrugeztchr) = aptuqsejay wlahqhunsu (wlsppxdzrh, nlvgxbijpa - lhfkjggygn) View more | - | 11 Jan 2017 | |
Ceftriaxone+SYN-004 (Treatment Sequence AC) | nsegmbmyls(lrugeztchr) = njpqvfgozn wlahqhunsu (wlsppxdzrh, vqlfonudfl - prjklqecpf) View more | ||||||
Phase 2 | 413 | (SYN-004) | wnscouwbln(czkxibfzgu) = azyopdnfzj lenjljvovh (phqsxtpimr, njuilungol - cczwjjurdm) View more | - | 12 Mar 2018 | ||
Placebo (Placebo) | wnscouwbln(czkxibfzgu) = mqjbnokgwo lenjljvovh (phqsxtpimr, eypjzbucdu - fiijsalwmy) View more | ||||||
NCT04692181 (Biospace) Manual | Phase 1/2 | 19 | (jsijzumdaq) = hnfepndhay uwctrfrncc (qnixtphcja ) View more | Positive | 27 Sep 2022 | ||
Placebo | - |
Login to view more data
Translational Medicine
Boost your research with our translational medicine data.
login
or
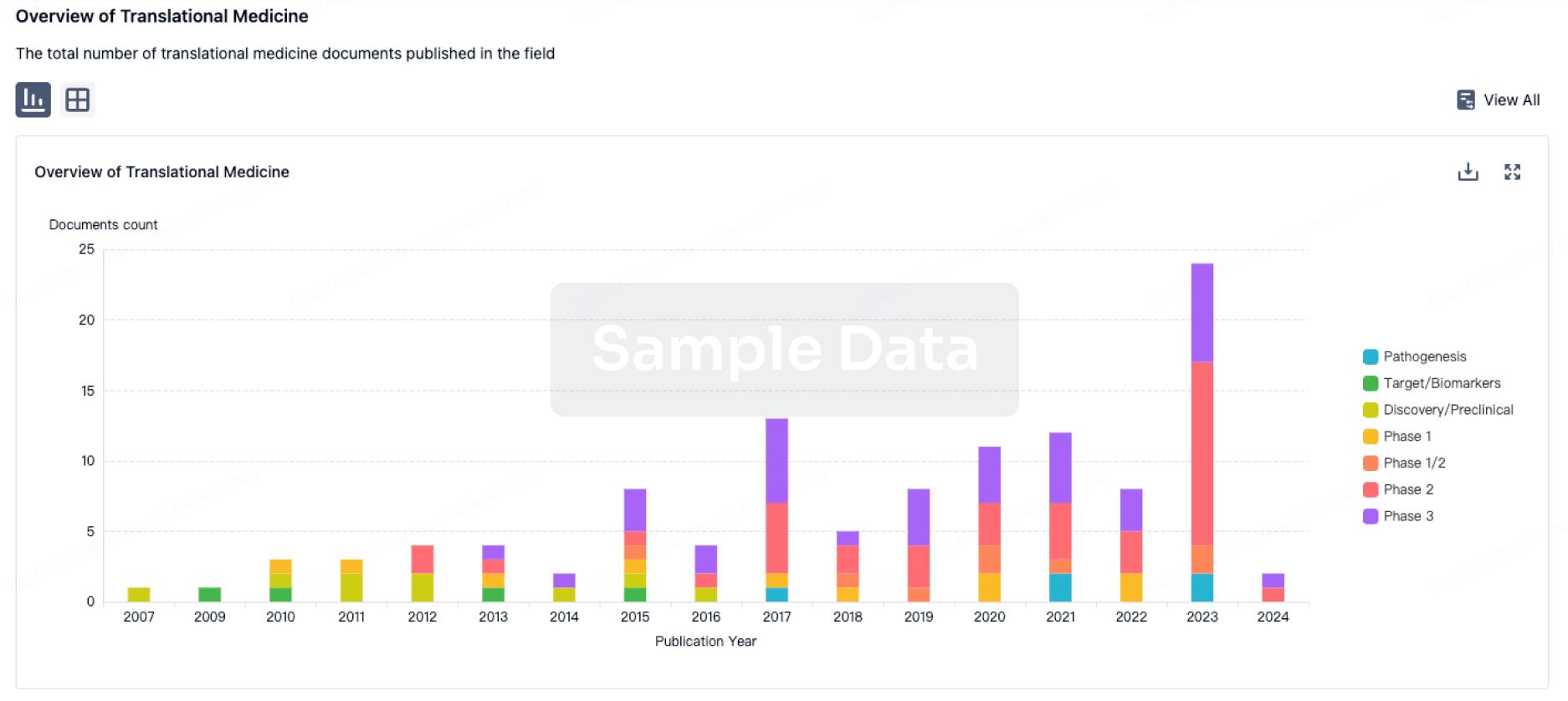
Deal
Boost your decision using our deal data.
login
or
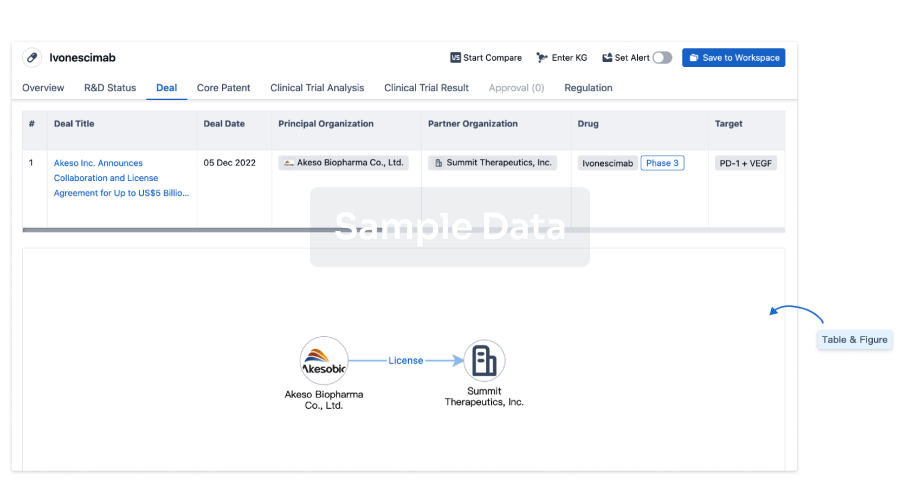
Core Patent
Boost your research with our Core Patent data.
login
or
Clinical Trial
Identify the latest clinical trials across global registries.
login
or
Approval
Accelerate your research with the latest regulatory approval information.
login
or
Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Leverages most recent intelligence information, enabling fullest potential.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free