Advancements in MPN Treatment: AJ1-10502, a Second-Generation JAK2 Inhibitor, Outperforms First-Generation Inhibitors in Preclinical Models
To address these issues, researchers have developed AJ1-10502, a novel type II JAK2 inhibitor with enhanced potency and selectivity. Using computational methods and structure-activity relationships, AJ1-10502 was identified and optimized for absorption, distribution, metabolism, and excretion. It showed significant JAK2 selectivity with minimal cross-reactivity compared to CHZ868.
In vitro studies indicated that AJ1-10502 effectively inhibited the proliferation of RUX-resistant cells, with an IC50 similar to that of RUX-sensitive cells. In vivo testing using a Jak2 knock-in/knock-out model demonstrated that AJ1-10502 could reduce leukocytosis and improve hematocrit and platelet levels, with spleen weight reductions comparable to genetic deletion of Jak2.
Importantly, AJ1-10502 was found to reduce the mutant allele fraction in peripheral blood and bone marrow, particularly within myeloid cell fractions, which was not observed with RUX. This suggests a targeted reduction in myeloid output. In a separate transplant model, AJ1-10502 confirmed its effects on leukocytosis, hematocrit, and spleen weight, and significantly reduced the mutant cell fraction in hematopoietic stem cells and progenitor populations.
A comparative study with CHZ868 showed that AJ1-10502 did not cause significant weight loss, indicating a comparable efficacy without systemic toxicity. Overall, AJ1-10502 is a potent and selective type II JAK2 inhibitor that offers improved efficacy and safety over previous JAK inhibitors. It has shown the potential to significantly reduce mutant cell fractions in vivo, which is a key advancement for the clinical development of type II JAK inhibitors for patients with myeloproliferative neoplasms.
How to Use Synapse Database to Search and Analyze Translational Medicine Data?
The transational medicine section of the Synapse database supports searches based on fields such as drug, target, and indication, covering the T0-T3 stages of translation. Additionally, it offers a historical conference search function as well as filtering options, view modes, translation services, and highlights summaries, providing you with a unique search experience.
Taking obesity as an example, select "obesity" under the indication category and click search to enter the Translational Medicine results list page. By clicking on the title, you can directly navigate to the original page.
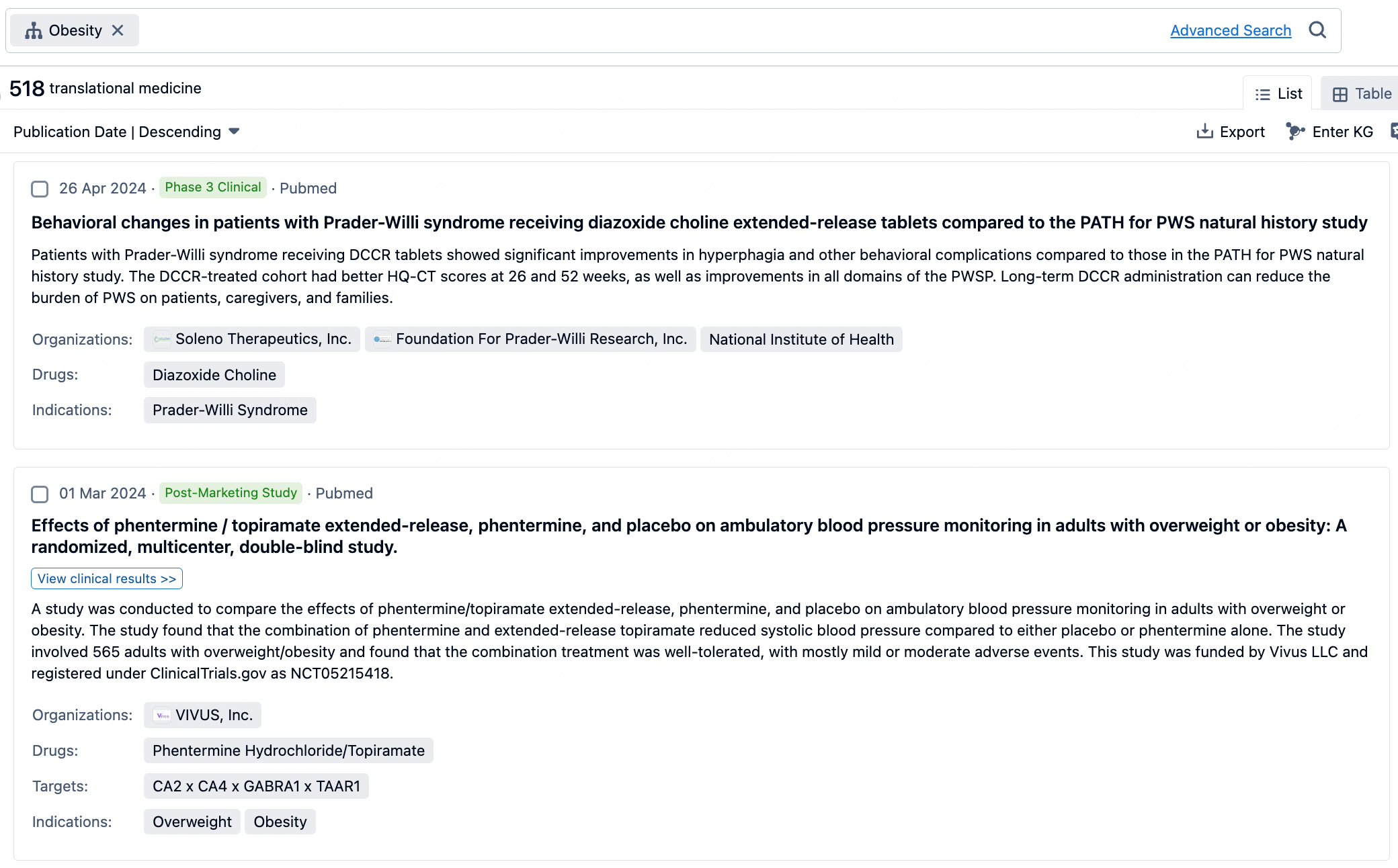
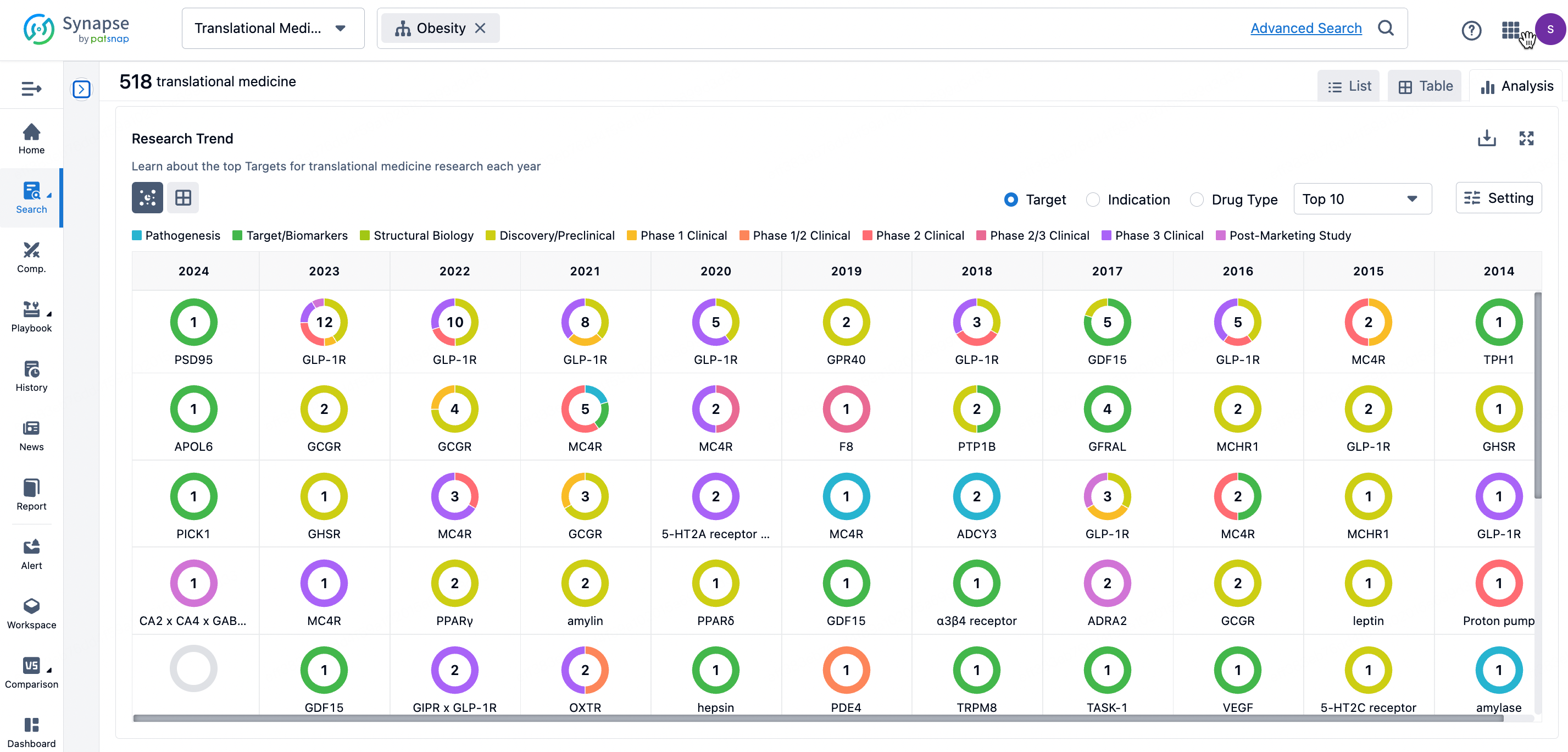
By clicking the analysis button, you can observe that GLP-1R treatment for obesity has gained significant attention over the past three years, with preclinical research still ongoing in 2023. Additionally, there are emerging potential targets, such as GDF15, among others.
Click on the image below to go directly to the Translational Medicine search interface.