Advancements in Targeted Therapy: The Role of AZD9291 in Overcoming EGFR Resistance in Lung Adenocarcinoma
AZD9291 is a third-generation, irreversible, and selective EGFR inhibitor designed to target both EGFR activating mutations and the resistance mutation T790M. It has been evaluated in vitro and in vivo using various cell lines with different EGFR mutations or wild-type EGFR. AZD9291 has demonstrated potent inhibition of EGFR phosphorylation in EGFRm+ and EGFRm+/T790M cell lines at low nanomolar concentrations, with minimal activity against wild-type EGFR. It also showed a significantly more potent inhibition of proliferation in mutant EGFR cell lines compared to wild-type in vitro.
In vivo studies have shown that once-daily oral dosing of AZD9291 at 5mg/kg led to substantial tumor regression in EGFRm+ and EGFRm+/T790M tumor models after 14 days of treatment. Furthermore, the same dosage was effective in causing significant shrinkage of EGFRm+ and EGFRm+/T790M transgenic mouse lung tumors. The tumor growth inhibition was associated with significant inhibition of EGFR phosphorylation and downstream signaling pathways.
Long-term treatment with AZD9291 resulted in a complete and sustained macroscopic response in xenograft tumor models, with no visible tumors after 40 days of dosing, and the effect was maintained beyond 100 days. Additionally, AZD9291 has shown potential to target tumors that have developed resistance to HER2-amplification, suggesting a broader application in TKI-resistant patients.
The preclinical data supports the potency and selectivity of AZD9291 against EGFR activating and resistance mutations while sparing wild-type EGFR, warranting further clinical investigation of AZD9291 for the treatment of advanced EGFR mutant lung adenocarcinoma.
How to Use Synapse Database to Search and Analyze Translational Medicine Data?
The transational medicine section of the Synapse database supports searches based on fields such as drug, target, and indication, covering the T0-T3 stages of translation. Additionally, it offers a historical conference search function as well as filtering options, view modes, translation services, and highlights summaries, providing you with a unique search experience.
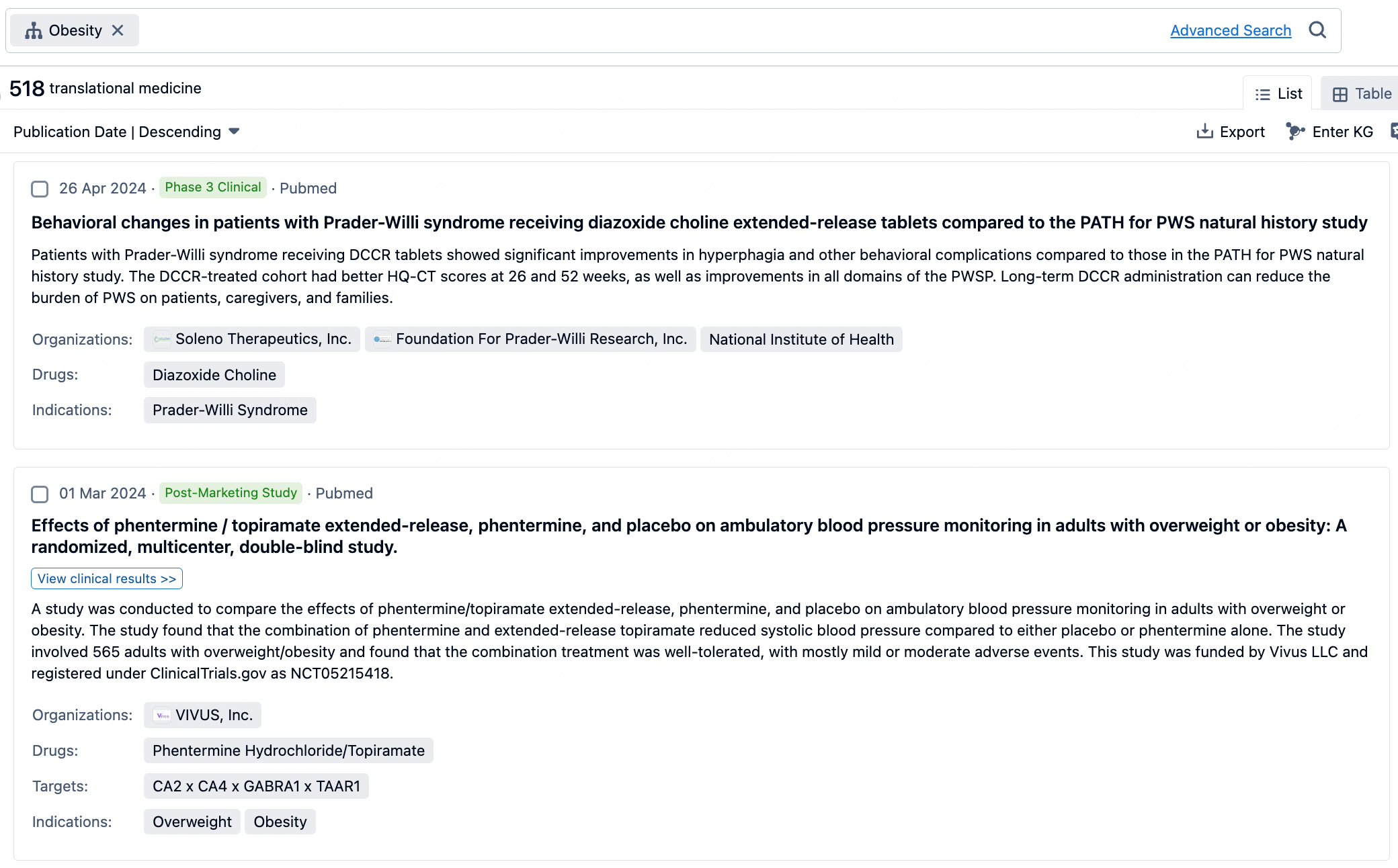
Taking obesity as an example, select "obesity" under the indication category and click search to enter the Translational Medicine results list page. By clicking on the title, you can directly navigate to the original page.
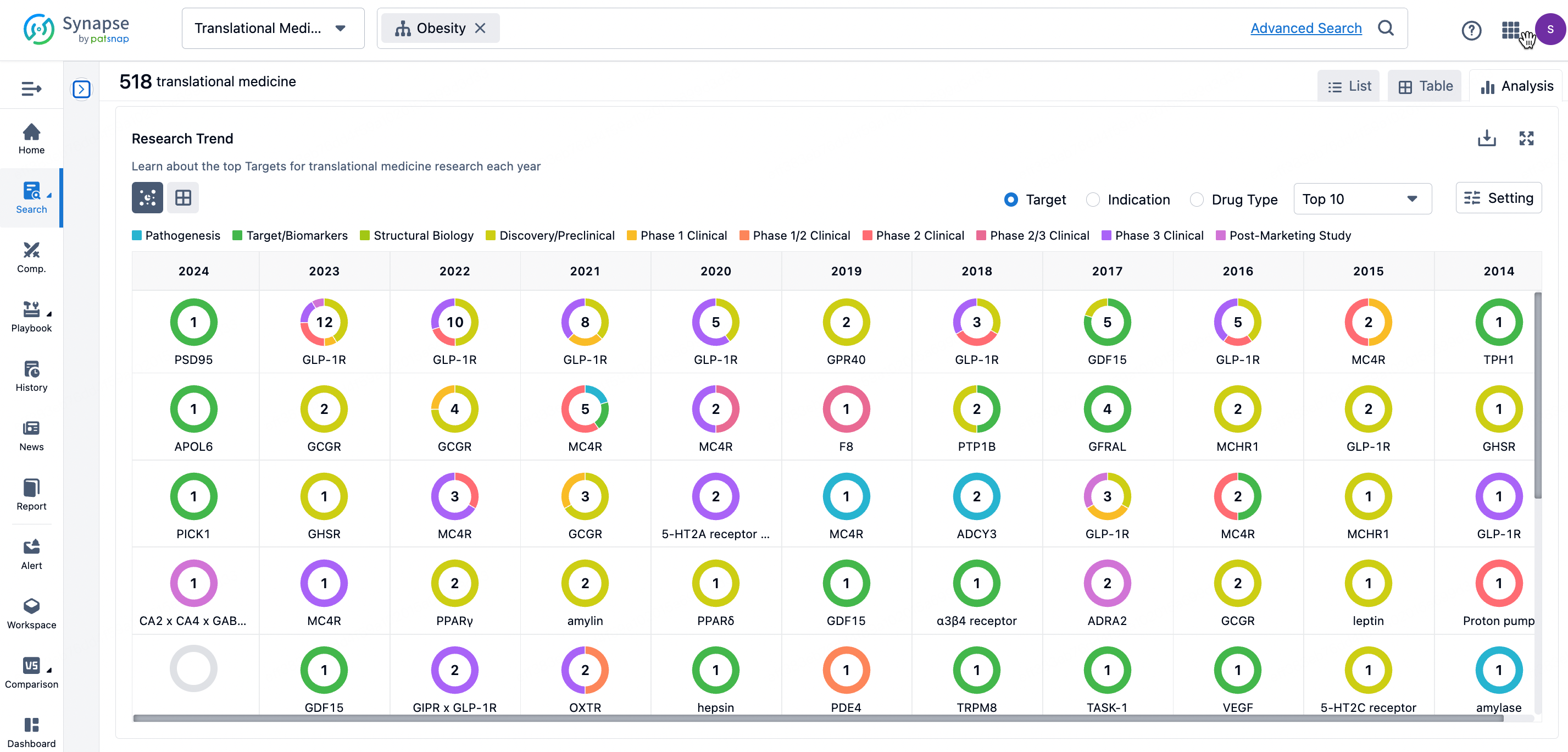
By clicking the analysis button, you can observe that GLP-1R treatment for obesity has gained significant attention over the past three years, with preclinical research still ongoing in 2023. Additionally, there are emerging potential targets, such as GDF15, among others.
Click on the image below to go directly to the Translational Medicine search interface.