Assessing the Safety and Efficacy of 225Ac-HuM195 in Cynomolgus Monkeys: Pharmacokinetics, Dosimetry, and Toxicity Insights
In assessing the toxicity of these nanogenerators, cynomolgus monkeys were utilized as subjects. The monoclonal antibody HuM195, which is intended for human clinical trials with (225)Ac, was used as the carrier, despite the absence of CD33 receptors in cynomolgus monkeys. In one study, two monkeys received a single intravenous dose of (225)Ac-HuM195 at 28 kBq/kg, which is close to the intended initial human dosage. Another experiment involved two animals receiving a cumulative dose of 377 kBq/kg through a dose escalation schedule of three increasing (225)Ac-HuM195 doses. The study monitored the half-life of (225)Ac in whole blood, the ratio of (225)Ac to its ultimate alpha-emitting daughter nuclide (213)Bi, the presence of monkey anti-HuM195 antibodies, hematologic indices, serum biochemistry, and clinical parameters. The monkeys were subjected to histopathological examination upon reaching toxic levels during the dose escalation.
The results showed that the half-life of (225)Ac-HuM195 in blood was 12 days, with 45% of the generated (213)Bi byproducts being cleared from the blood. No production of monkey anti-HuM195 antibodies was detected. A dosage of approximately 28 kBq/kg of (225)Ac did not exhibit toxicity after six months, while a cumulative dose of around 377 kBq/kg led to severe toxicity. In the cumulative dosing schedule, doses of approximately 37 kBq/kg showed no toxicity after six weeks. After administering approximately 130 kBq/kg, no toxicity was observed for 13 weeks. However, mild anemia and increased levels of blood urea nitrogen and creatinine were noted 28 weeks post the second dose administration. Upon an additional 185 kBq/kg dose, clinical toxicity became evident. The monkeys were euthanized 13 and 19 weeks after the third dose, with histopathological findings indicating primarily renal tubular damage and interstitial fibrosis.
The conclusion drawn from the study suggests that high doses of (225)Ac nanogenerators could lead to renal toxicity and anemia. The extended blood half-life and the lack of target cell antigens in cynomolgus monkeys might increase toxicity compared to human use. Thus, a starting dose of at least 28 kBq/kg is proposed as a potentially safe dosage for human trials, with the necessity for close monitoring of hematologic and renal functions.
How to Use Synapse Database to Search and Analyze Translational Medicine Data?
The transational medicine section of the Synapse database supports searches based on fields such as drug, target, and indication, covering the T0-T3 stages of translation. Additionally, it offers a historical conference search function as well as filtering options, view modes, translation services, and highlights summaries, providing you with a unique search experience.
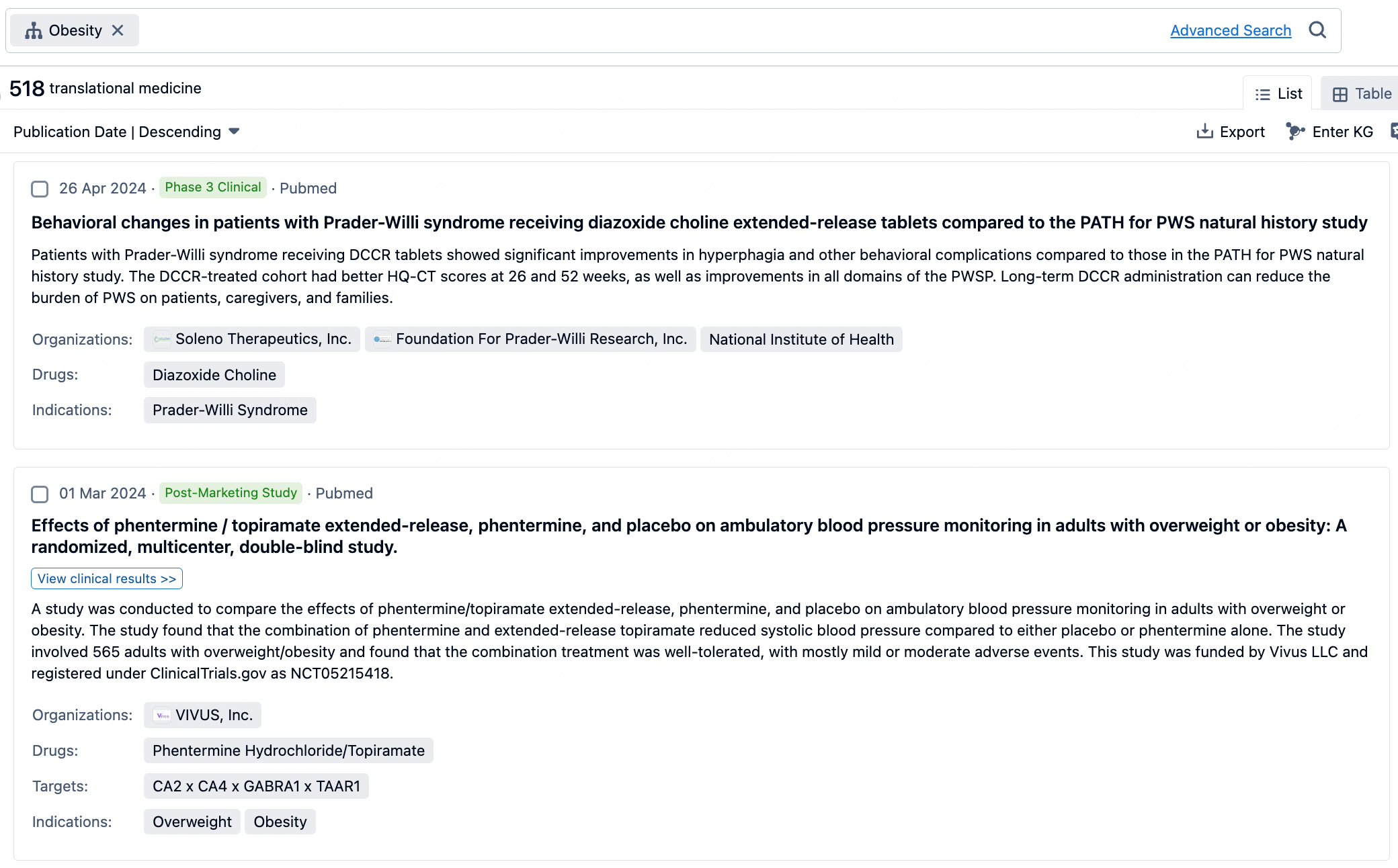
Taking obesity as an example, select "obesity" under the indication category and click search to enter the Translational Medicine results list page. By clicking on the title, you can directly navigate to the original page.
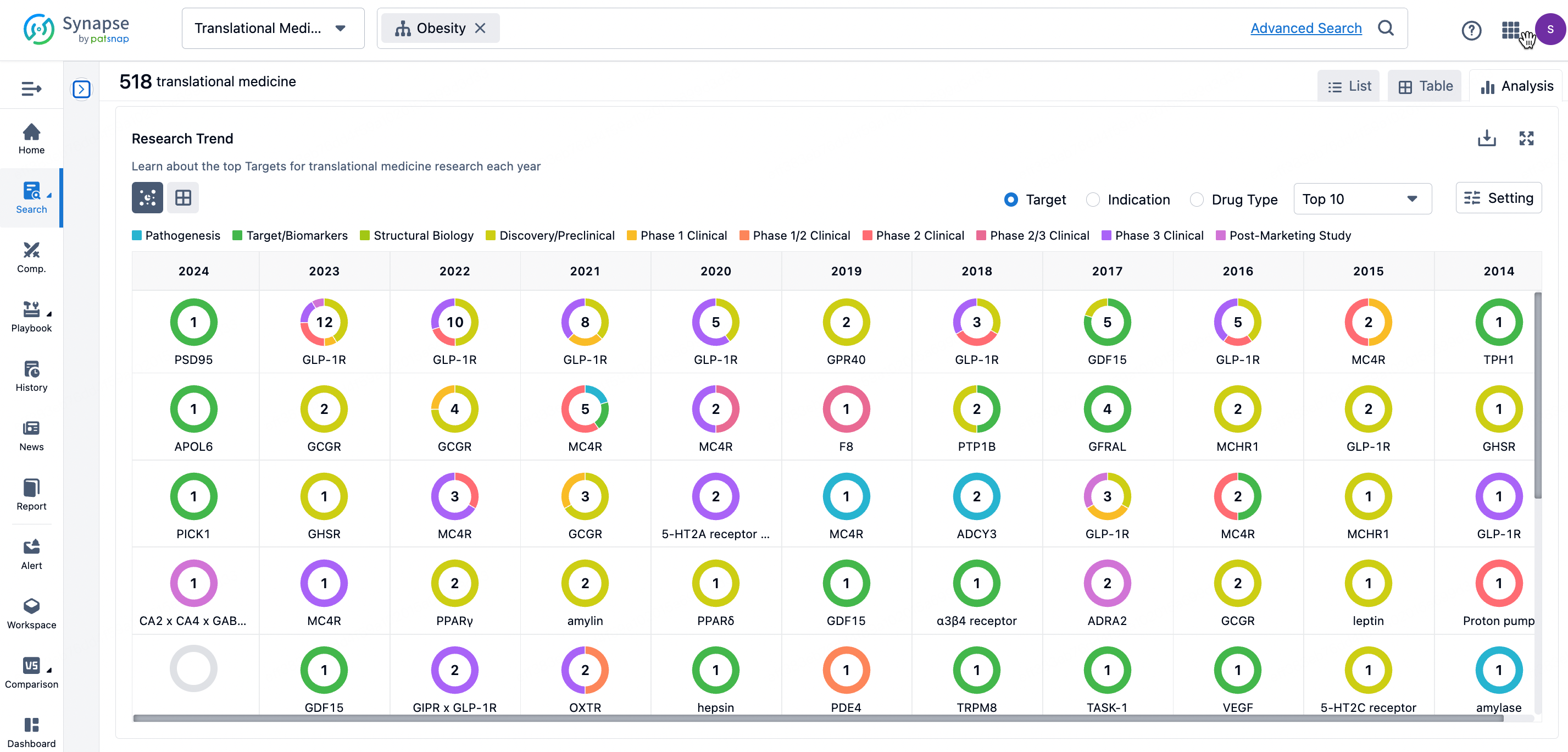
By clicking the analysis button, you can observe that GLP-1R treatment for obesity has gained significant attention over the past three years, with preclinical research still ongoing in 2023. Additionally, there are emerging potential targets, such as GDF15, among others.
Click on the image below to go directly to the Translational Medicine search interface.