Exploring the Potential of HDP-101: A BCMA-Targeted Antibody-Drug Conjugate for Multiple Myeloma
This study focuses on antibody-targeted amanitin conjugates (ATACs), a novel class of ADCs that use amanitin as the toxic payload. Amanitin, a well-known toxin from the amatoxin family, binds to eukaryotic RNA polymerase II and inhibits cellular transcription at low concentrations, regardless of the cell's proliferation status.
The study evaluated the specificity and efficacy of HDP-101, an ATAC that targets BCMA (B cell maturation antigen), a protein expressed on B cell lineage cells, especially plasma blasts and plasma cells, and is highly expressed in malignant plasma cells, making it an ideal target for multiple myeloma.
In vitro tests showed that HDP-101 had significant cytotoxic effects on BCMA-positive myeloma cell lines and primary CD138+ cells from refractory myeloma patients, even at low epitope densities. Importantly, no toxicity was observed in control cells without BCMA expression or in myeloma cells treated with a non-target control antibody loaded with amanitin.
In mouse models of human myeloma, HDP-101 induced dose-dependent tumor regression, with complete remissions observed after single intravenous doses ranging from 0.1 mg/kg to 4.0 mg/kg.
Safety studies in Cynomolgus monkeys demonstrated good tolerability and a favorable therapeutic index with sequential doses of HDP-101. Hematology and clinical chemistry parameters remained largely unaffected, with only a transient increase in liver enzymes and lactate dehydrogenase. The serum half-life of HDP-101 was approximately 12 days, with the free toxin detectable at very low levels.
In conclusion, HDP-101, an anti-BCMA ATAC, effectively delivered targeted cytotoxic drug to BCMA-positive myeloma cell lines, leading to an efficient anti-tumor response both in vitro and in vivo, with good tolerability in non-human primates. Amanitin-based ADCs represent a promising new approach for the treatment of multiple myeloma, with a distinct mode of action that may overcome drug resistance and improve patient outcomes. A first-in-human trial for HDP-101 is anticipated to commence in 2018.
How to Use Synapse Database to Search and Analyze Translational Medicine Data?
The transational medicine section of the Synapse database supports searches based on fields such as drug, target, and indication, covering the T0-T3 stages of translation. Additionally, it offers a historical conference search function as well as filtering options, view modes, translation services, and highlights summaries, providing you with a unique search experience.
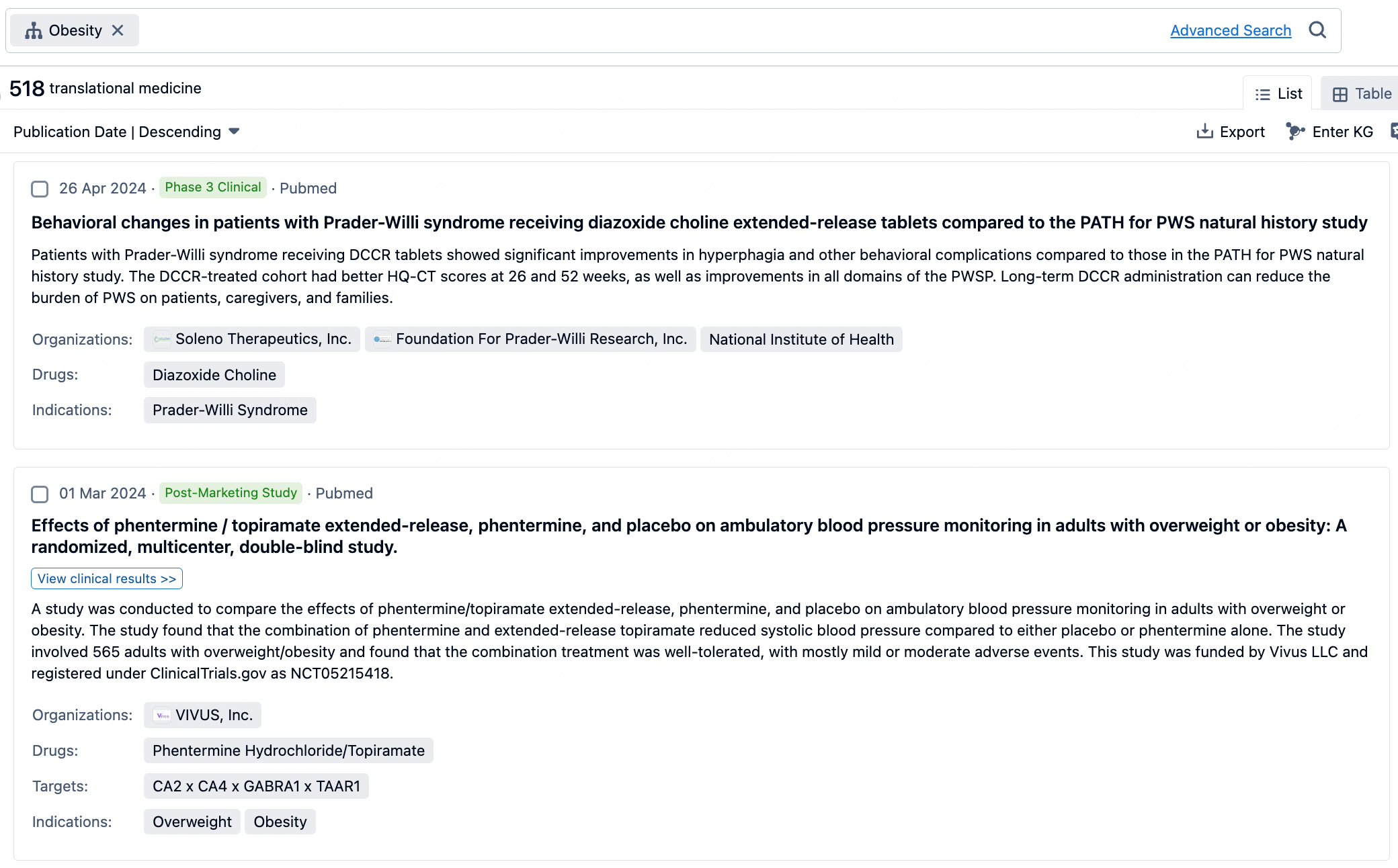
Taking obesity as an example, select "obesity" under the indication category and click search to enter the Translational Medicine results list page. By clicking on the title, you can directly navigate to the original page.
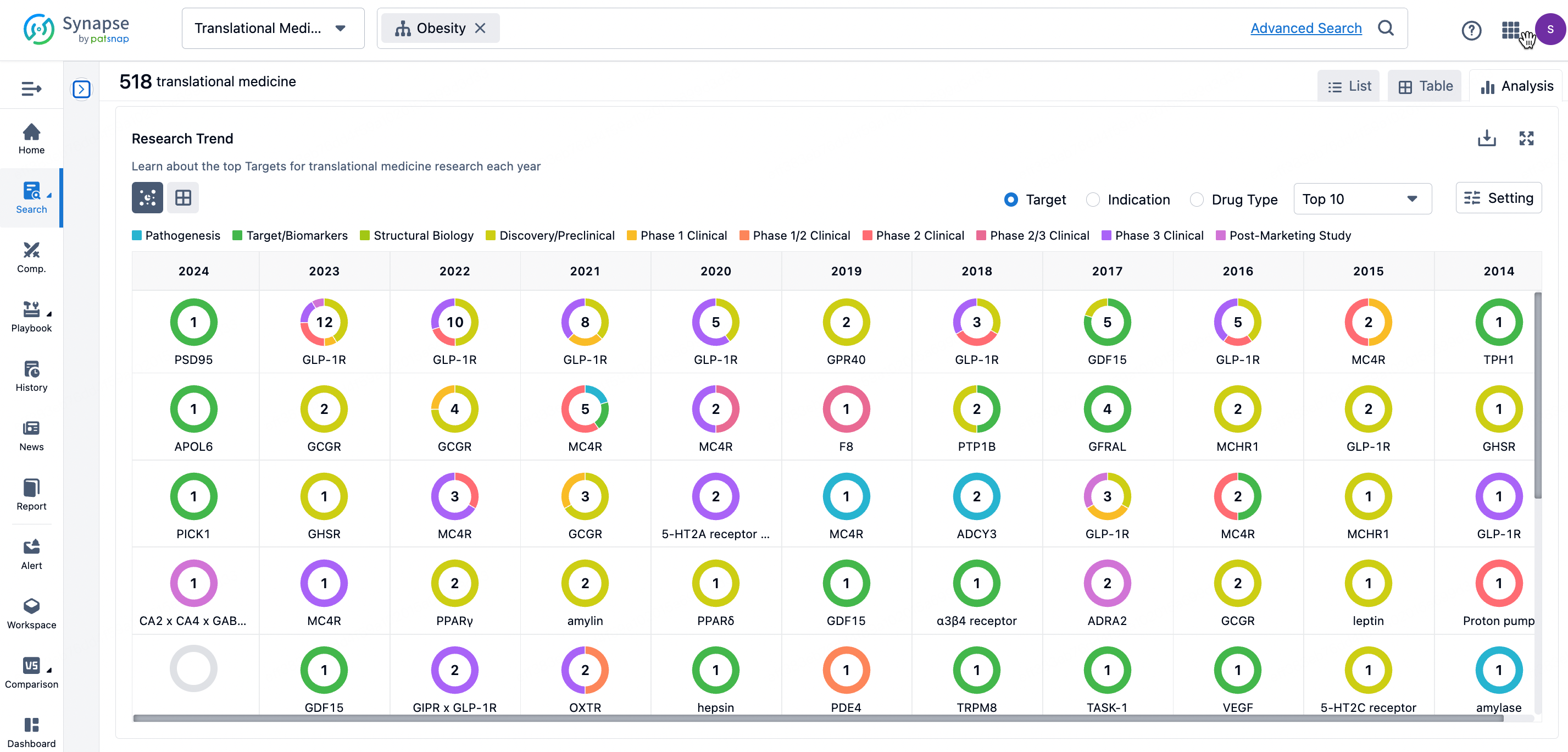
By clicking the analysis button, you can observe that GLP-1R treatment for obesity has gained significant attention over the past three years, with preclinical research still ongoing in 2023. Additionally, there are emerging potential targets, such as GDF15, among others.
Click on the image below to go directly to the Translational Medicine search interface.