Harnessing the Power of T Cells: ISB 1342 as a Breakthrough Therapy for Relapsed and Refractory Multiple Myeloma
The antibody was meticulously engineered to include a single chain variable fragment (scFv) that binds to CD3ε on T cells and a Fab arm that targets CD38, ensuring compatibility with daratumumab. This dual engagement strategy redirects T cells to eliminate CD38-expressing tumor cells, offering a distinct advantage over existing CD38-targeting therapies.
In laboratory tests, ISB 1342 demonstrated remarkable efficacy, killing a wide array of CD38-positive tumor cell lines with significantly greater potency than daratumumab. Notably, it proved effective against tumor cells with low to intermediate CD38 expression, a group that daratumumab struggled to impact. Furthermore, ISB 1342 maintained its effectiveness when combined with daratumumab, irrespective of whether it was used sequentially or concurrently. The presence of soluble CD38 or glucocorticoids did not diminish ISB 1342's killing power.
The antibody was also designed with a double LALA mutation to reduce binding to Fcγ receptors and C1q, thereby minimizing Fc-mediated effector functions. This design choice underscores the importance of T cell engagement and activation in ISB 1342's mechanism of action. In vitro, ISB 1342 showed a high degree of target specificity and did not trigger T cell activation without the presence of CD38-positive cells. Importantly, ISB 1342-induced tumor cell killing was not accompanied by T cell fratricide.
In vivo studies using a Daudi tumor model further confirmed ISB 1342's superior potency. Unlike daratumumab, which only partially controlled tumor growth, ISB 1342 led to complete tumor eradication when administered intravenously at a weekly dose of 0.5 mg/kg. A control molecule lacking the relevant CD38 binder failed to control tumor growth, highlighting the specificity and efficacy of ISB 1342.
Post-treatment analysis revealed a significant increase in the release of Granzyme A and B, TNF-alpha, and CXCL-10 in the tumor microenvironment, indicative of an enhanced anti-tumor immune response associated with ISB 1342's in vivo efficacy.
The superior potency of ISB 1342 compared to daratumumab supports its ongoing clinical development for the treatment of multiple myeloma patients who have exhausted other therapeutic options.
How to Use Synapse Database to Search and Analyze Translational Medicine Data?
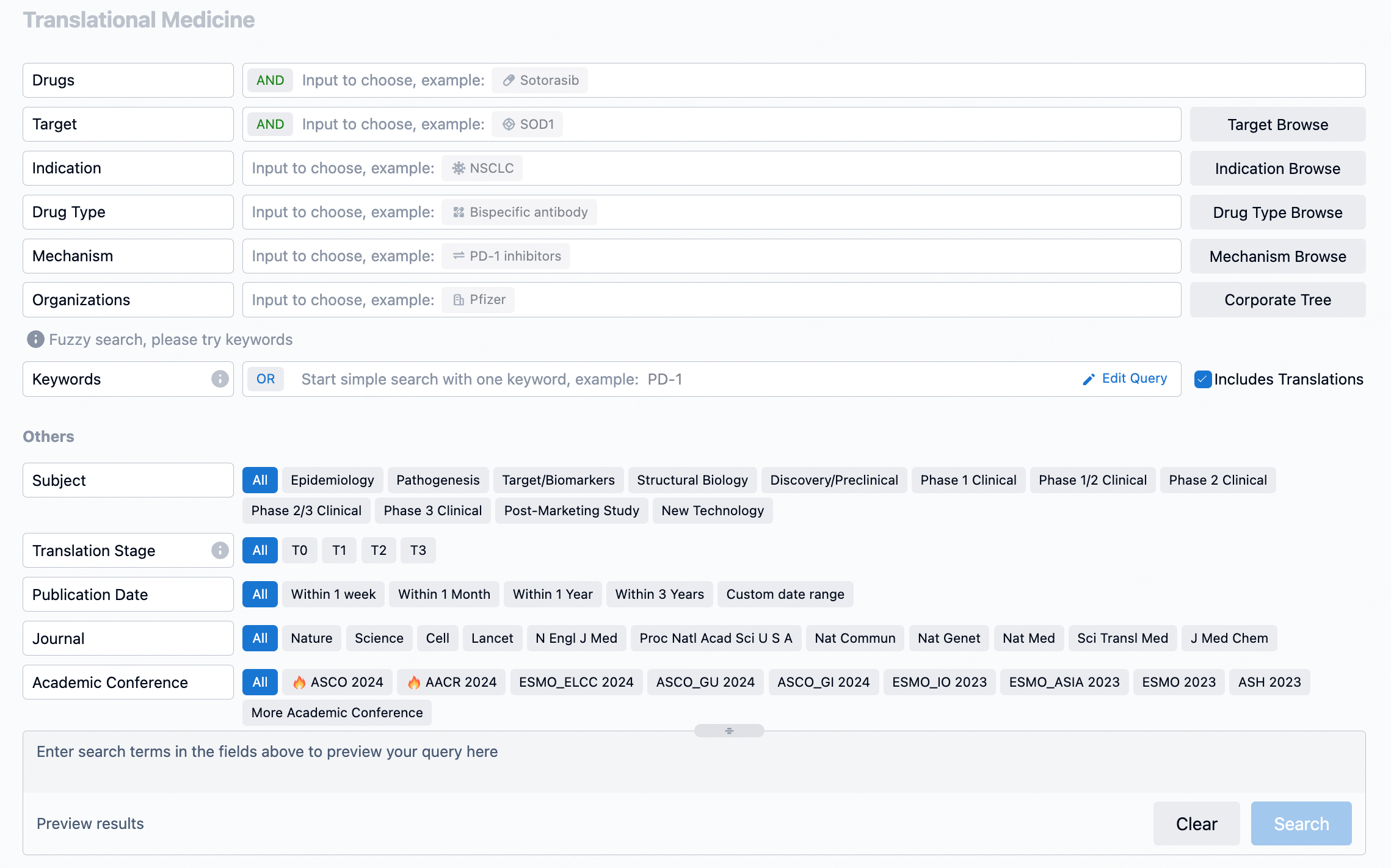
The transational medicine section of the Synapse database supports searches based on fields such as drug, target, and indication, covering the T0-T3 stages of translation. Additionally, it offers a historical conference search function as well as filtering options, view modes, translation services, and highlights summaries, providing you with a unique search experience.
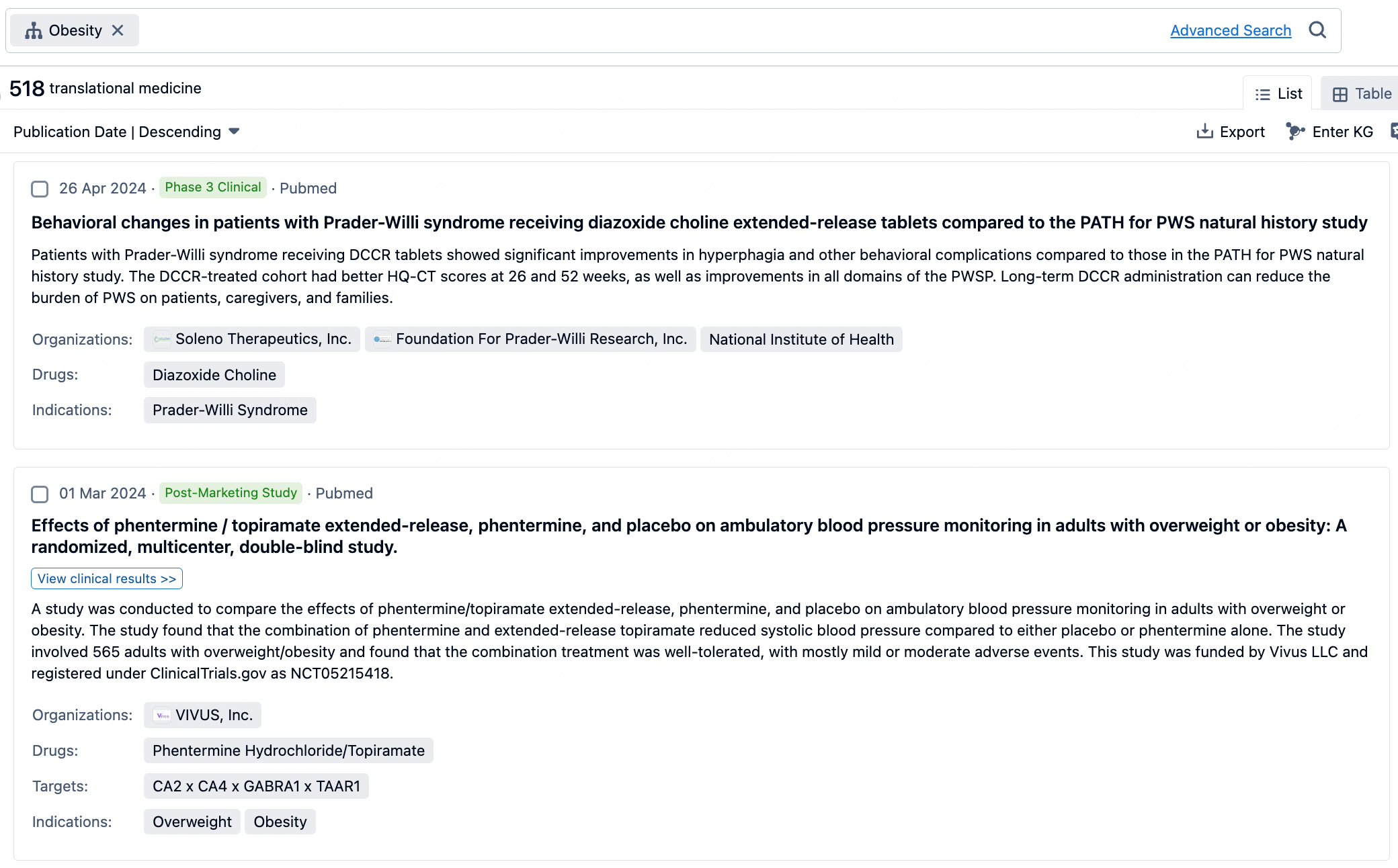
Taking obesity as an example, select "obesity" under the indication category and click search to enter the Translational Medicine results list page. By clicking on the title, you can directly navigate to the original page.
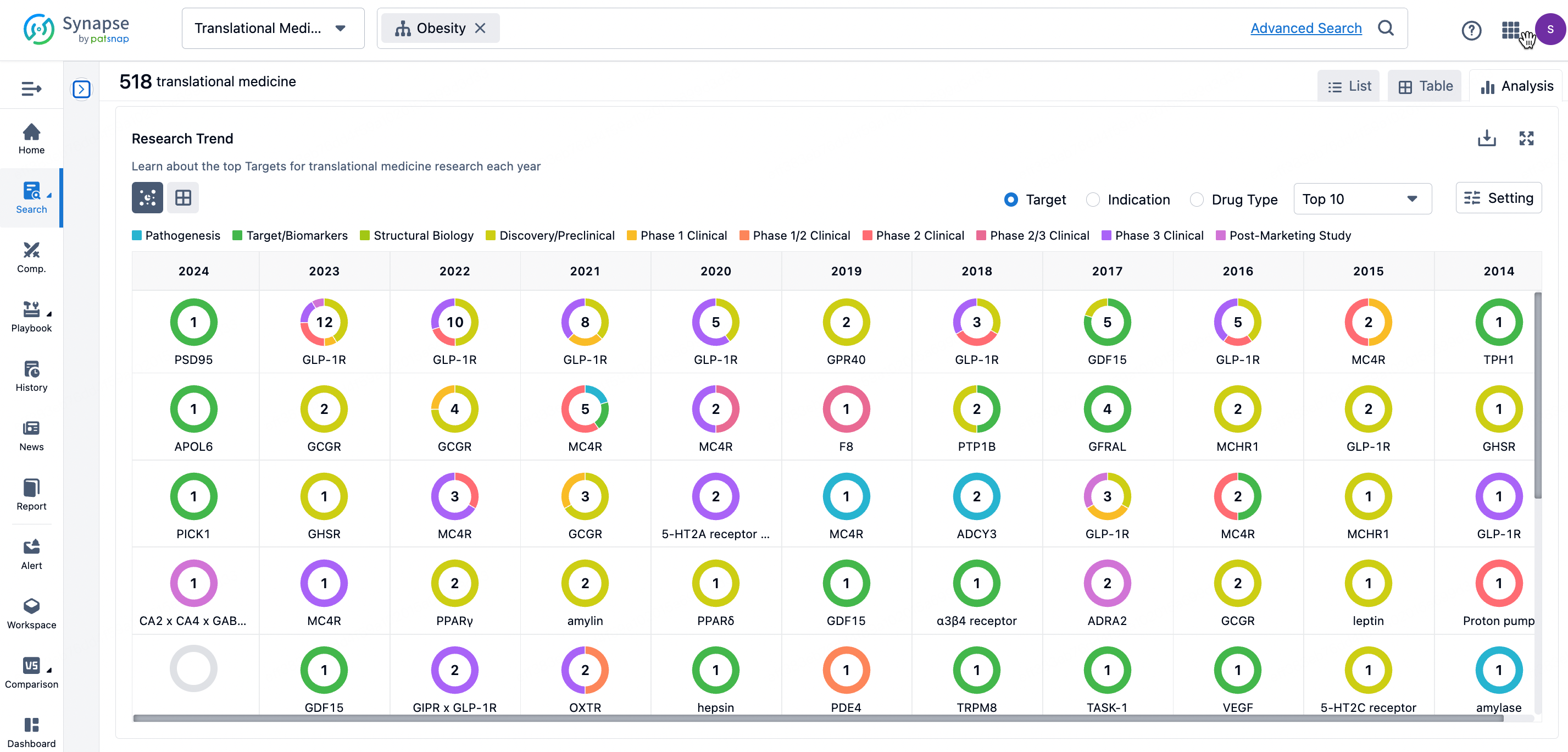
By clicking the analysis button, you can observe that GLP-1R treatment for obesity has gained significant attention over the past three years, with preclinical research still ongoing in 2023. Additionally, there are emerging potential targets, such as GDF15, among others.
Click on the image below to go directly to the Translational Medicine search interface.