Request Demo
How to Choose Between CHO Cells and Microbial Systems for Biologics
7 May 2025
When embarking on the complex journey of biologics production, one of the critical decisions involves choosing the right expression system. Among the various options available, Chinese Hamster Ovary (CHO) cells and microbial systems stand out as two prevalent choices. Each system has unique attributes, benefits, and limitations, making the selection process highly dependent on the specific requirements of your biologics project.
CHO cells are a well-established mammalian cell line used extensively in the production of therapeutic proteins. They are particularly valued for their ability to perform complex post-translational modifications, such as glycosylation, which are often essential for the activity, stability, and efficacy of biologic drugs. This makes CHO cells an ideal choice for producing monoclonal antibodies and other therapeutic proteins that require human-like modifications.
One of the key advantages of CHO cells is their adaptability to various growth conditions and the capability to be cultured in serum-free and suspension media, which simplifies downstream processing and reduces costs. Furthermore, regulatory agencies have a long history of approving CHO cell-derived products, providing a smoother pathway through the regulatory landscape.
However, CHO cells are not without their challenges. They typically have longer doubling times compared to microbial systems, resulting in slower production cycles. The culture of CHO cells can also be more expensive, given the requirement for complex media and stringent growth conditions. Additionally, the scale-up process can be more intricate, requiring significant expertise and resources.
On the other hand, microbial systems, such as Escherichia coli or yeast, offer several compelling advantages, particularly in terms of speed and cost. These systems have rapid growth rates, allowing for quick protein production and high yields, which are particularly beneficial during the early stages of development when speed is essential to meet tight timelines.
Microbial systems are also known for their simplicity and cost-effectiveness. They require less complex media and conditions, significantly reducing operational costs. Furthermore, genetic manipulations in microbial systems are generally more straightforward, allowing for faster development and optimization of production strains.
However, microbial systems come with their own set of limitations. The lack of post-translational modifications similar to those in human proteins can be a significant drawback. While certain yeast strains can perform some glycosylation, the patterns often differ substantially from those in humans, which can affect the functionality and immunogenicity of the final product. Moreover, proteins expressed in microbial systems often require additional purification steps to ensure proper folding and activity, which can complicate the downstream processing.
Choosing between CHO cells and microbial systems ultimately hinges on several critical factors. The nature of the biologic being produced is paramount—complex proteins requiring specific post-translational modifications may necessitate the use of CHO cells, despite the higher costs and longer production times. Conversely, simpler proteins that do not require such modifications can benefit from the cost-effectiveness and speed of microbial systems.
Additionally, the stage of development and production scale are important considerations. Early-stage research and small-scale production may favor microbial systems due to their rapid turnaround and lower costs. In contrast, larger-scale commercial production, particularly for products requiring regulatory approval, might justify the investment in CHO cells for their proven track record and ability to produce complex, human-like proteins.
In conclusion, both CHO cells and microbial systems offer distinct advantages and challenges. A thorough assessment of the specific needs of your biologics project, including the nature of the protein, timeline, budget, and regulatory considerations, is essential to making an informed decision. Engaging with experienced professionals and leveraging their expertise can provide valuable insights and guidance, ensuring the chosen expression system aligns with your project's goals and facilitates the successful development of high-quality biologics.
CHO cells are a well-established mammalian cell line used extensively in the production of therapeutic proteins. They are particularly valued for their ability to perform complex post-translational modifications, such as glycosylation, which are often essential for the activity, stability, and efficacy of biologic drugs. This makes CHO cells an ideal choice for producing monoclonal antibodies and other therapeutic proteins that require human-like modifications.
One of the key advantages of CHO cells is their adaptability to various growth conditions and the capability to be cultured in serum-free and suspension media, which simplifies downstream processing and reduces costs. Furthermore, regulatory agencies have a long history of approving CHO cell-derived products, providing a smoother pathway through the regulatory landscape.
However, CHO cells are not without their challenges. They typically have longer doubling times compared to microbial systems, resulting in slower production cycles. The culture of CHO cells can also be more expensive, given the requirement for complex media and stringent growth conditions. Additionally, the scale-up process can be more intricate, requiring significant expertise and resources.
On the other hand, microbial systems, such as Escherichia coli or yeast, offer several compelling advantages, particularly in terms of speed and cost. These systems have rapid growth rates, allowing for quick protein production and high yields, which are particularly beneficial during the early stages of development when speed is essential to meet tight timelines.
Microbial systems are also known for their simplicity and cost-effectiveness. They require less complex media and conditions, significantly reducing operational costs. Furthermore, genetic manipulations in microbial systems are generally more straightforward, allowing for faster development and optimization of production strains.
However, microbial systems come with their own set of limitations. The lack of post-translational modifications similar to those in human proteins can be a significant drawback. While certain yeast strains can perform some glycosylation, the patterns often differ substantially from those in humans, which can affect the functionality and immunogenicity of the final product. Moreover, proteins expressed in microbial systems often require additional purification steps to ensure proper folding and activity, which can complicate the downstream processing.
Choosing between CHO cells and microbial systems ultimately hinges on several critical factors. The nature of the biologic being produced is paramount—complex proteins requiring specific post-translational modifications may necessitate the use of CHO cells, despite the higher costs and longer production times. Conversely, simpler proteins that do not require such modifications can benefit from the cost-effectiveness and speed of microbial systems.
Additionally, the stage of development and production scale are important considerations. Early-stage research and small-scale production may favor microbial systems due to their rapid turnaround and lower costs. In contrast, larger-scale commercial production, particularly for products requiring regulatory approval, might justify the investment in CHO cells for their proven track record and ability to produce complex, human-like proteins.
In conclusion, both CHO cells and microbial systems offer distinct advantages and challenges. A thorough assessment of the specific needs of your biologics project, including the nature of the protein, timeline, budget, and regulatory considerations, is essential to making an informed decision. Engaging with experienced professionals and leveraging their expertise can provide valuable insights and guidance, ensuring the chosen expression system aligns with your project's goals and facilitates the successful development of high-quality biologics.
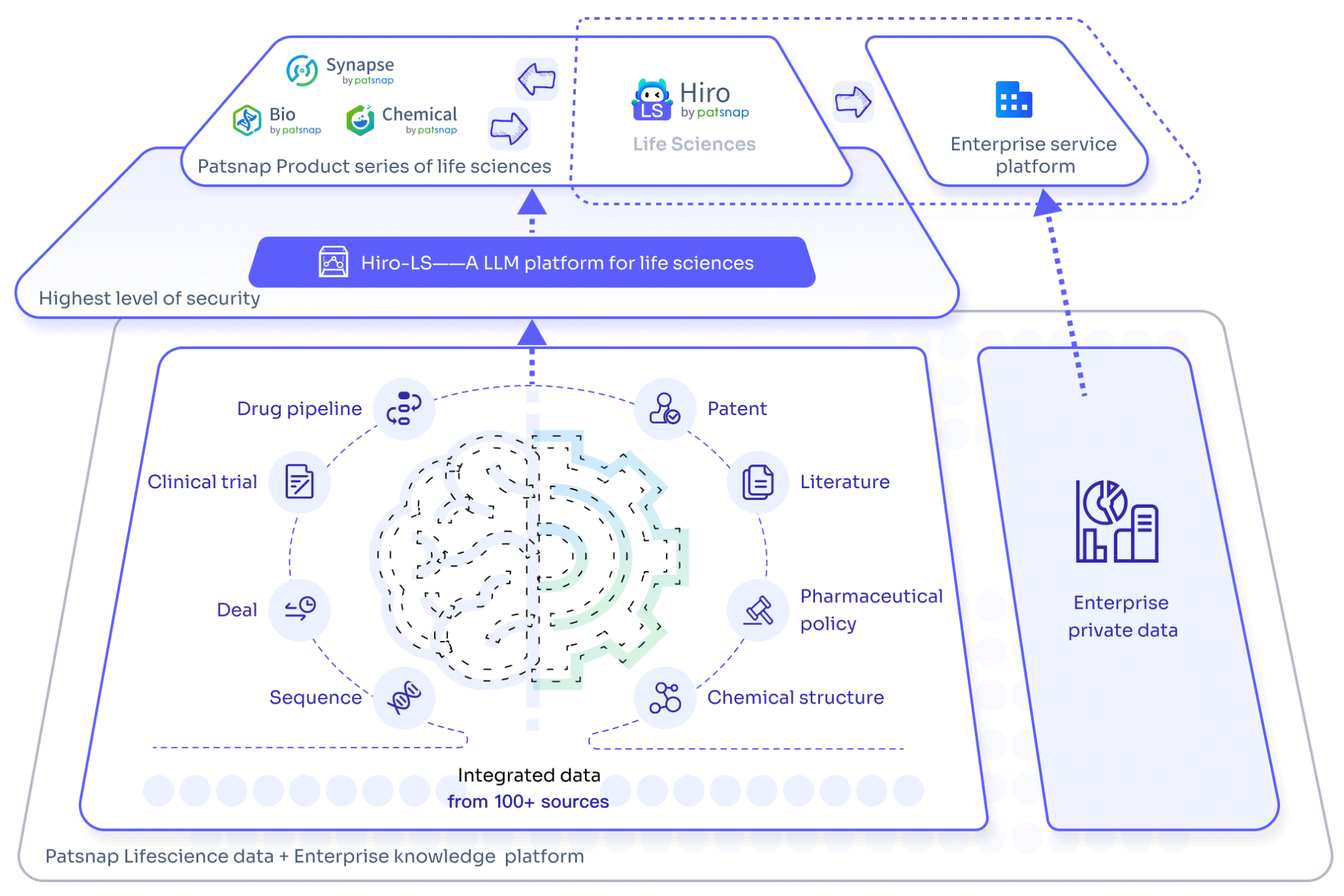
For an experience with the large-scale biopharmaceutical model Hiro-LS, please click here for a quick and free trial of its features!
AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.