Targeting RET Mutations: The Promise of DS-5010 in Cancer Therapy
DS-5010, a novel oral small-molecule RET inhibitor, has been found to be highly potent and specific against RET and gatekeeper-mutated RET (RET-GKm), with minimal KDR activity. In vitro assays revealed that DS-5010 significantly inhibits RET and platelet-derived growth factor receptor (PDGFR) alpha/beta at low concentrations, with a half-maximal inhibitory concentration (IC50) in the single-digit nano-molar range for RET and RET-GKm, even in the presence of high ATP levels. In contrast, its IC50 against KDR was substantially higher.
In vivo models demonstrated DS-5010's effectiveness in inducing tumor regression at specific dosages, unlike FDA-approved MTKIs which showed no significant antitumor effect in a model with RET-GKm mutation. Furthermore, DS-5010 was effective in an NSCLC xenograft model with the RET-CCDC6 fusion gene.
Resistance to FDA-approved MTKIs was addressed by establishing resistant clones through prolonged exposure to cabozantinib, which all possessed the V804E mutation in the RET kinase domain. DS-5010 was able to inhibit the proliferation of these resistant clones effectively, whereas FDA-approved MTKIs showed weak inhibitory effects.
The study concludes that DS-5010 exhibits strong in vitro and in vivo activities against RET and RET-GKm mutations, suggesting its potential as a targeted therapy for cancers with RET gene rearrangements. It also shows promise in combating MTKI-resistant cells. Investigational new drug-enabling studies for DS-5010 are currently underway.
Reference: Kaneta Y, Komatsu T, Miyamoto M, et al. Preclinical characterization and antitumor efficacy of DS-5010, a highly potent and selective RET inhibitor [abstract]. Mol Cancer Ther 2018;17(1 Suppl):Abstract nr B173.
How to Use Synapse Database to Search and Analyze Translational Medicine Data?
The transational medicine section of the Synapse database supports searches based on fields such as drug, target, and indication, covering the T0-T3 stages of translation. Additionally, it offers a historical conference search function as well as filtering options, view modes, translation services, and highlights summaries, providing you with a unique search experience.
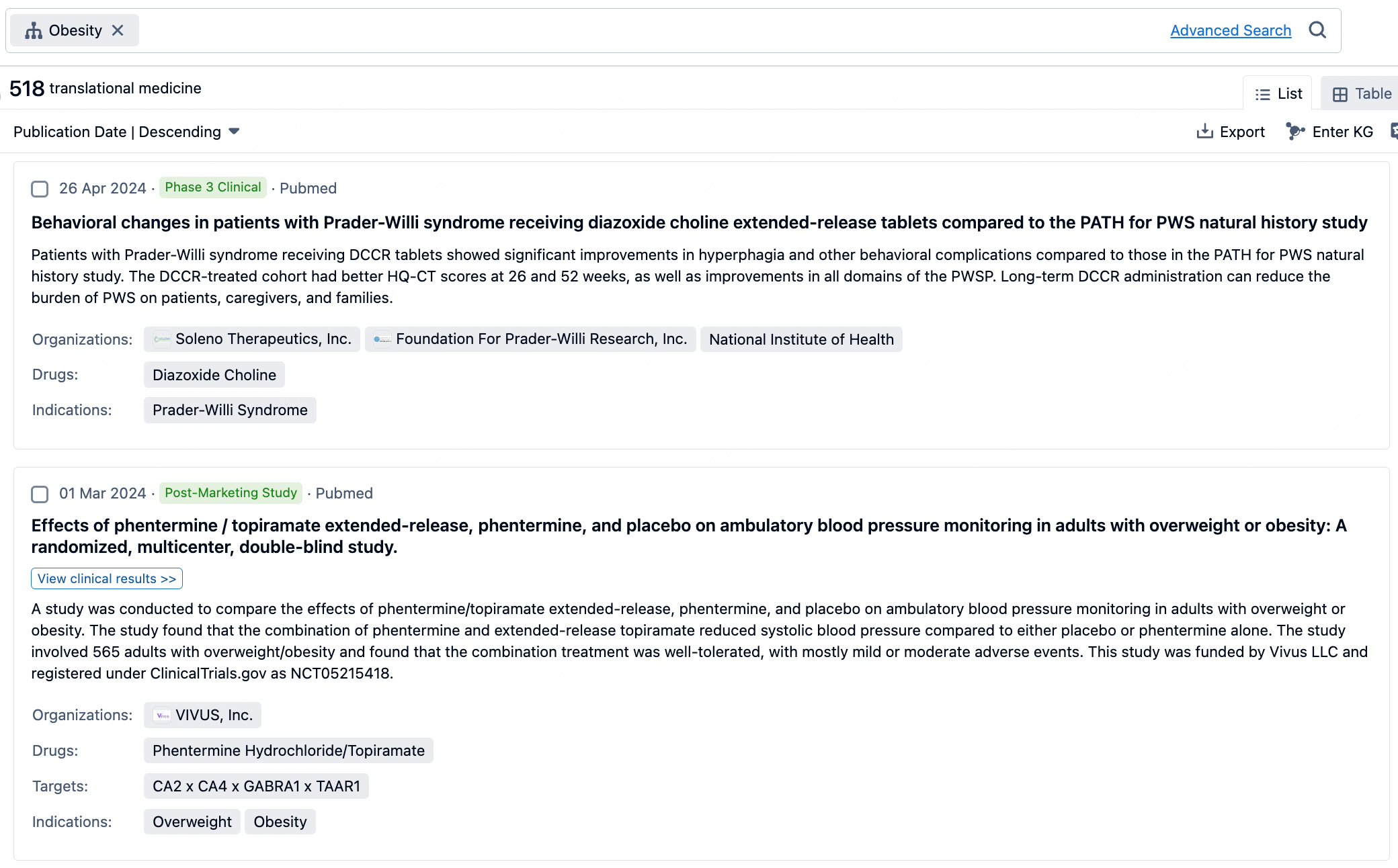
Taking obesity as an example, select "obesity" under the indication category and click search to enter the Translational Medicine results list page. By clicking on the title, you can directly navigate to the original page.
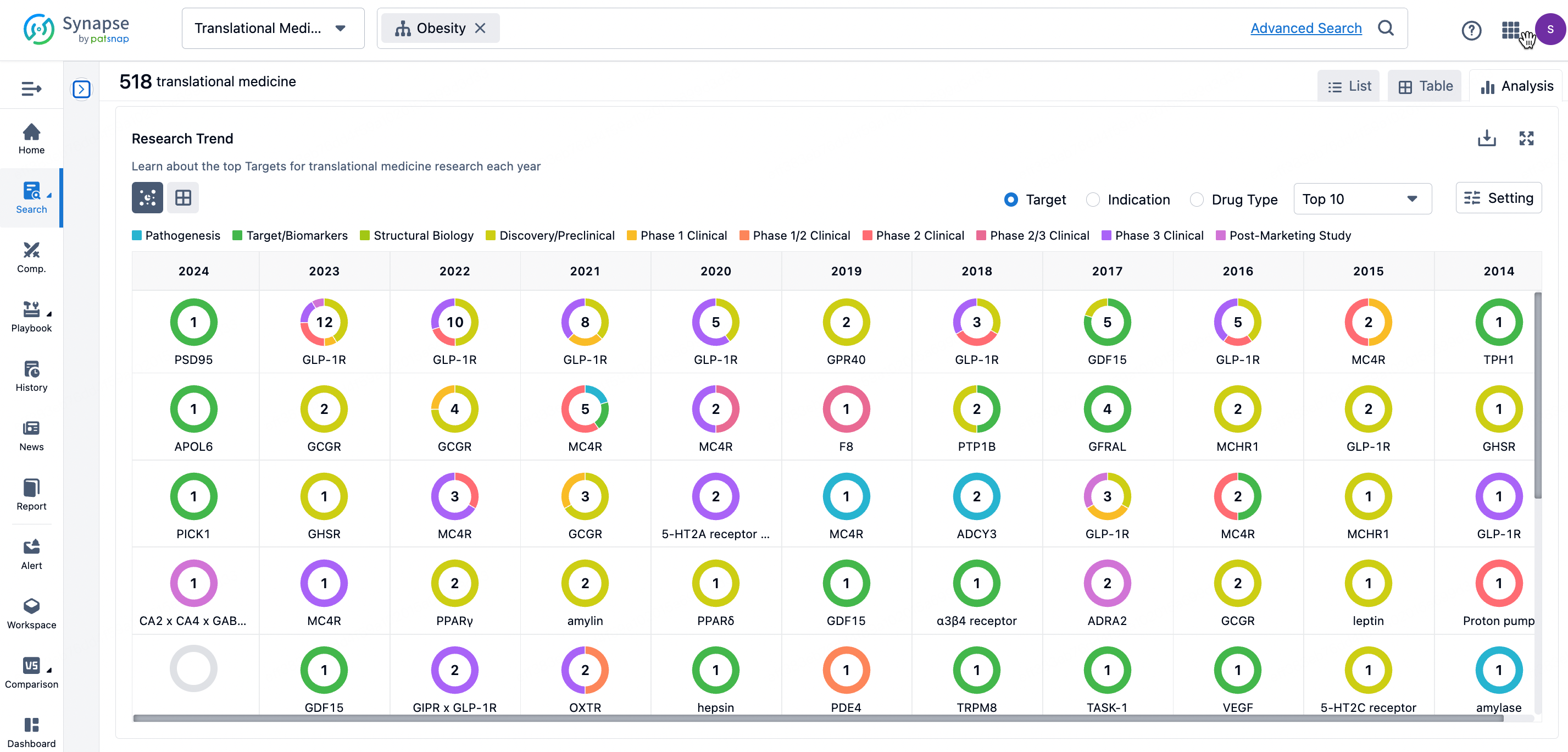
By clicking the analysis button, you can observe that GLP-1R treatment for obesity has gained significant attention over the past three years, with preclinical research still ongoing in 2023. Additionally, there are emerging potential targets, such as GDF15, among others.
Click on the image below to go directly to the Translational Medicine search interface.