Unlocking the Potential of HPN217: Targeting BCMA for Innovative Multiple Myeloma Therapy
A novel treatment, HPN217, is a tri-specific T cell activating construct (TriTAC) that includes binding domains for human BCMA, human serum albumin (HSA), and the T cell receptor (TCR) complex. This construct is a stable single polypeptide with a molecular weight of approximately 53 kDa, produced by CHO cells. The simultaneous binding of BCMA on MM cells and CD3 on T cells triggers T cell activation and the destruction of target MM cells.
The inclusion of an HSA binding domain in HPN217 is a unique strategy that extends the serum half-life of the molecule and provides it with a small size and flexibility, distinguishing it from other CD3-based bispecific T cell engaging molecules that use Fc-engineering.
The binding affinities of HPN217 for recombinant human BCMA, HSA, and CD3ε were determined to be 5.5 nM, 6 nM, and 17 nM, respectively, using biolayer interferometry. Flow cytometry confirmed the construct's binding to native targets on the cell surface. In vitro pharmacological activity was evaluated using T cell-dependent cellular cytotoxicity (TDCC) assays, demonstrating dose-dependent and BCMA-dependent cytotoxicity with EC50 values ranging from 0.05 to 0.7 nM.
The in vivo properties of HPN217 were assessed in xenograft models and a pharmacokinetic study in cynomolgus monkeys. The construct showed dose-dependent growth suppression against certain MM and lymphoma models with low BCMA expression levels. In the pharmacokinetic study, HPN217 displayed linear behavior and a serum half-life of 64 to 85 hours, with stability and intactness maintained up to 3 weeks in vivo.
The preclinical and nonclinical data indicate that HPN217 is an effective therapeutic candidate with the potential for a convenient dosing schedule. A phase 1 clinical trial is planned to assess the safety and efficacy of HPN217 in patients with RRMM.
How to Use Synapse Database to Search and Analyze Translational Medicine Data?
The transational medicine section of the Synapse database supports searches based on fields such as drug, target, and indication, covering the T0-T3 stages of translation. Additionally, it offers a historical conference search function as well as filtering options, view modes, translation services, and highlights summaries, providing you with a unique search experience.
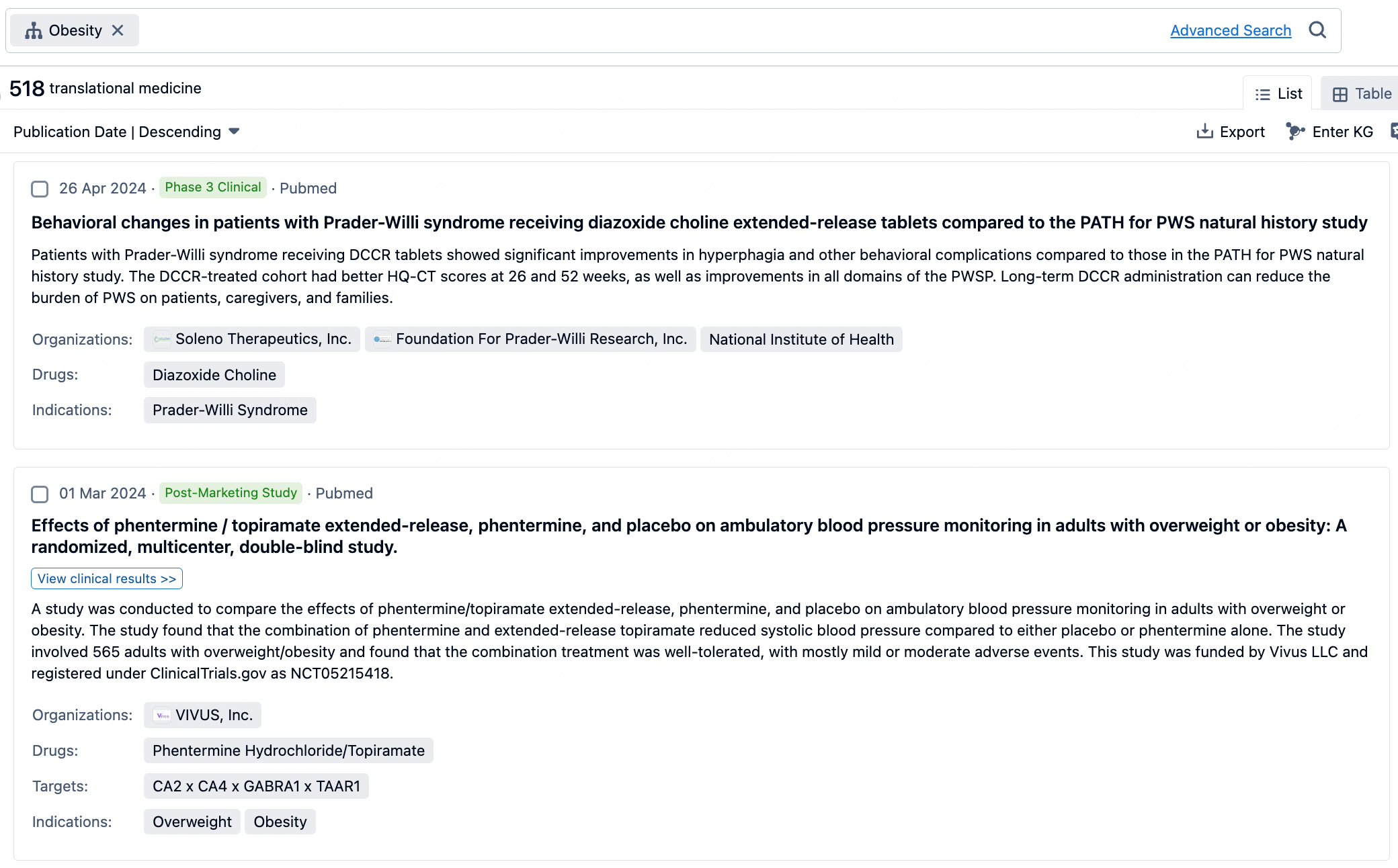
Taking obesity as an example, select "obesity" under the indication category and click search to enter the Translational Medicine results list page. By clicking on the title, you can directly navigate to the original page.
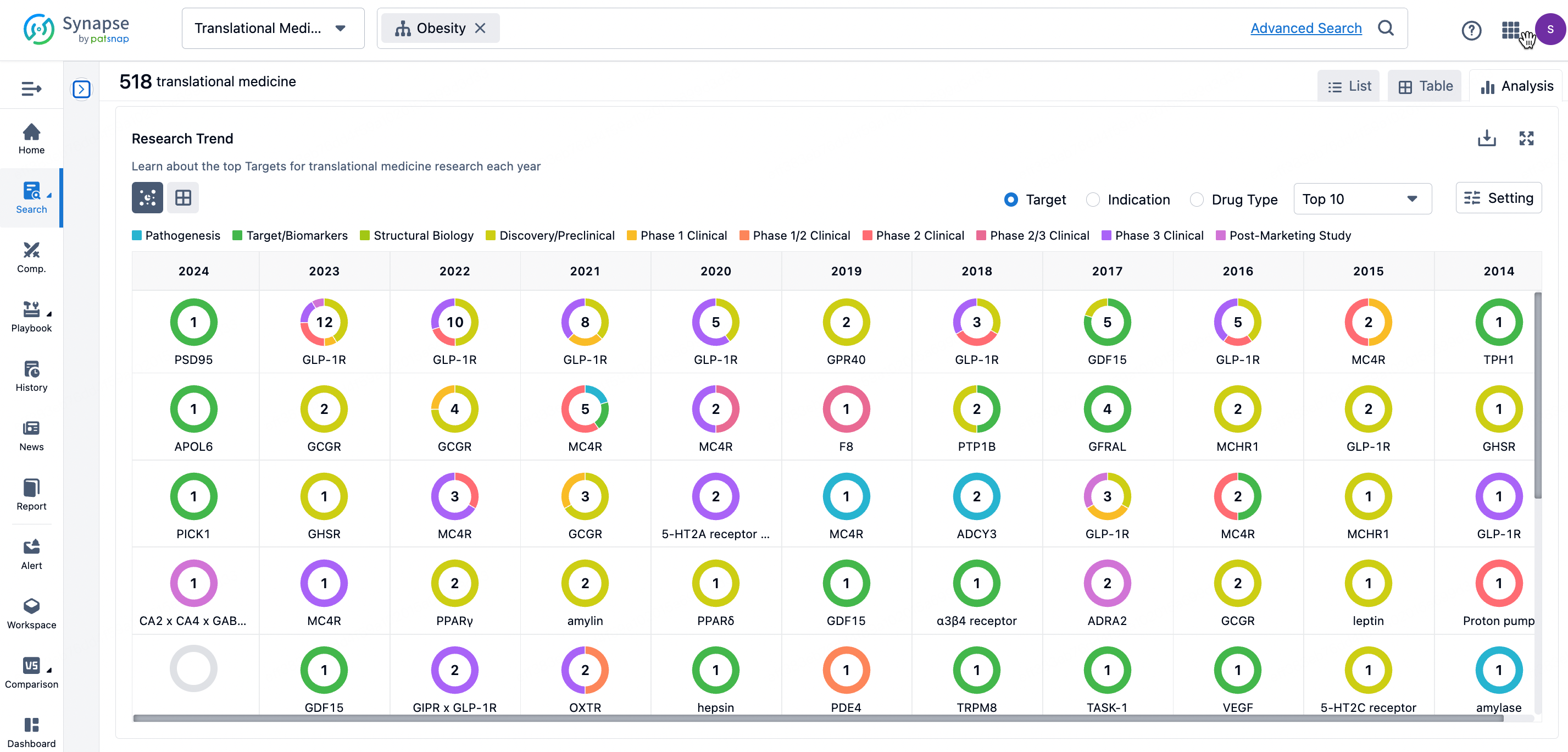
By clicking the analysis button, you can observe that GLP-1R treatment for obesity has gained significant attention over the past three years, with preclinical research still ongoing in 2023. Additionally, there are emerging potential targets, such as GDF15, among others.
Click on the image below to go directly to the Translational Medicine search interface.