Request Demo
What are Stathmin inhibitors and how do they work?
26 June 2024
Stathmin inhibitors have gained considerable attention in recent years due to their potential applications in cancer therapy. Stathmin, a phosphoprotein involved in microtubule dynamics, plays a pivotal role in cell division. By regulating the assembly and disassembly of microtubules, Stathmin ensures proper mitotic spindle formation which is crucial for accurate chromosome segregation. Dysregulation of Stathmin activity has been implicated in various cancers, making it a compelling target for therapeutic intervention. This article delves into the mechanisms of action, applications, and the burgeoning research surrounding Stathmin inhibitors.
Stathmin inhibitors primarily work by disrupting the normal function of Stathmin in microtubule dynamics. Under physiological conditions, Stathmin binds to tubulin, sequestering it and preventing its polymerization into microtubules. This action is critical during the mitotic spindle formation where accurate chromosome segregation is essential for cell division.
In cancer cells, Stathmin is often overexpressed, leading to aberrant microtubule dynamics and uncontrolled cell proliferation. Stathmin inhibitors aim to counteract this by either reducing Stathmin expression or inhibiting its activity. These inhibitors function through various mechanisms such as small interfering RNA (siRNA) that targets Stathmin mRNA for degradation, small molecules that bind to Stathmin and impede its interaction with tubulin, or through post-translational modifications that alter its activity.
The inhibition of Stathmin activity results in the stabilization of microtubules, thereby disrupting the mitotic spindle apparatus. This disruption triggers a cascade of events leading to cell cycle arrest and ultimately, apoptosis or programmed cell death. By selectively targeting cancer cells with high Stathmin levels, these inhibitors can minimize damage to normal cells, thereby reducing the side effects commonly associated with traditional chemotherapy.
The primary application of Stathmin inhibitors has been in the realm of oncology. Research has demonstrated that many cancers, including breast cancer, ovarian cancer, and leukemia, exhibit elevated levels of Stathmin. In these cancers, Stathmin overexpression correlates with poor prognosis and increased resistance to conventional therapies. Stathmin inhibitors have shown promise in preclinical studies by sensitizing cancer cells to existing treatments, thereby enhancing their efficacy.
In breast cancer, for instance, studies have shown that Stathmin inhibitors can significantly reduce tumor growth and metastatic potential. Similarly, in ovarian cancer, these inhibitors have been found to enhance the effectiveness of platinum-based chemotherapy, a standard treatment for this type of cancer. Moreover, in leukemia, Stathmin inhibitors have demonstrated the ability to induce apoptosis in drug-resistant cancer cells, offering a potential lifeline for patients with refractory disease.
Beyond oncology, Stathmin inhibitors are also being explored for their potential in treating neurodegenerative diseases. Recent studies suggest that Stathmin plays a role in neuronal plasticity and axonal regeneration. In conditions such as Alzheimer’s disease and spinal cord injuries, where microtubule stability is compromised, Stathmin inhibitors may offer therapeutic benefits by promoting microtubule stabilization and supporting neuronal repair mechanisms.
Despite the promising preclinical results, the clinical translation of Stathmin inhibitors faces several challenges. These include issues related to the delivery of these inhibitors to tumor sites, potential off-target effects, and the development of resistance mechanisms by cancer cells. Ongoing research is focused on overcoming these hurdles through the development of more selective inhibitors, advanced delivery systems, and combination therapies that can enhance the efficacy and safety profile of Stathmin inhibitors.
In conclusion, Stathmin inhibitors represent a novel and promising class of therapeutic agents with the potential to revolutionize cancer treatment and beyond. By targeting the microtubule dynamics crucial for cell division, these inhibitors offer a targeted approach to combat cancers characterized by Stathmin overexpression. While significant challenges remain, the ongoing advancements in this field hold great promise for the development of more effective and less toxic cancer therapies. As research continues to unravel the complexities of Stathmin regulation, the therapeutic landscape for Stathmin inhibitors is poised for exciting developments in the near future.
Stathmin inhibitors primarily work by disrupting the normal function of Stathmin in microtubule dynamics. Under physiological conditions, Stathmin binds to tubulin, sequestering it and preventing its polymerization into microtubules. This action is critical during the mitotic spindle formation where accurate chromosome segregation is essential for cell division.
In cancer cells, Stathmin is often overexpressed, leading to aberrant microtubule dynamics and uncontrolled cell proliferation. Stathmin inhibitors aim to counteract this by either reducing Stathmin expression or inhibiting its activity. These inhibitors function through various mechanisms such as small interfering RNA (siRNA) that targets Stathmin mRNA for degradation, small molecules that bind to Stathmin and impede its interaction with tubulin, or through post-translational modifications that alter its activity.
The inhibition of Stathmin activity results in the stabilization of microtubules, thereby disrupting the mitotic spindle apparatus. This disruption triggers a cascade of events leading to cell cycle arrest and ultimately, apoptosis or programmed cell death. By selectively targeting cancer cells with high Stathmin levels, these inhibitors can minimize damage to normal cells, thereby reducing the side effects commonly associated with traditional chemotherapy.
The primary application of Stathmin inhibitors has been in the realm of oncology. Research has demonstrated that many cancers, including breast cancer, ovarian cancer, and leukemia, exhibit elevated levels of Stathmin. In these cancers, Stathmin overexpression correlates with poor prognosis and increased resistance to conventional therapies. Stathmin inhibitors have shown promise in preclinical studies by sensitizing cancer cells to existing treatments, thereby enhancing their efficacy.
In breast cancer, for instance, studies have shown that Stathmin inhibitors can significantly reduce tumor growth and metastatic potential. Similarly, in ovarian cancer, these inhibitors have been found to enhance the effectiveness of platinum-based chemotherapy, a standard treatment for this type of cancer. Moreover, in leukemia, Stathmin inhibitors have demonstrated the ability to induce apoptosis in drug-resistant cancer cells, offering a potential lifeline for patients with refractory disease.
Beyond oncology, Stathmin inhibitors are also being explored for their potential in treating neurodegenerative diseases. Recent studies suggest that Stathmin plays a role in neuronal plasticity and axonal regeneration. In conditions such as Alzheimer’s disease and spinal cord injuries, where microtubule stability is compromised, Stathmin inhibitors may offer therapeutic benefits by promoting microtubule stabilization and supporting neuronal repair mechanisms.
Despite the promising preclinical results, the clinical translation of Stathmin inhibitors faces several challenges. These include issues related to the delivery of these inhibitors to tumor sites, potential off-target effects, and the development of resistance mechanisms by cancer cells. Ongoing research is focused on overcoming these hurdles through the development of more selective inhibitors, advanced delivery systems, and combination therapies that can enhance the efficacy and safety profile of Stathmin inhibitors.
In conclusion, Stathmin inhibitors represent a novel and promising class of therapeutic agents with the potential to revolutionize cancer treatment and beyond. By targeting the microtubule dynamics crucial for cell division, these inhibitors offer a targeted approach to combat cancers characterized by Stathmin overexpression. While significant challenges remain, the ongoing advancements in this field hold great promise for the development of more effective and less toxic cancer therapies. As research continues to unravel the complexities of Stathmin regulation, the therapeutic landscape for Stathmin inhibitors is poised for exciting developments in the near future.
How to obtain the latest development progress of all targets?
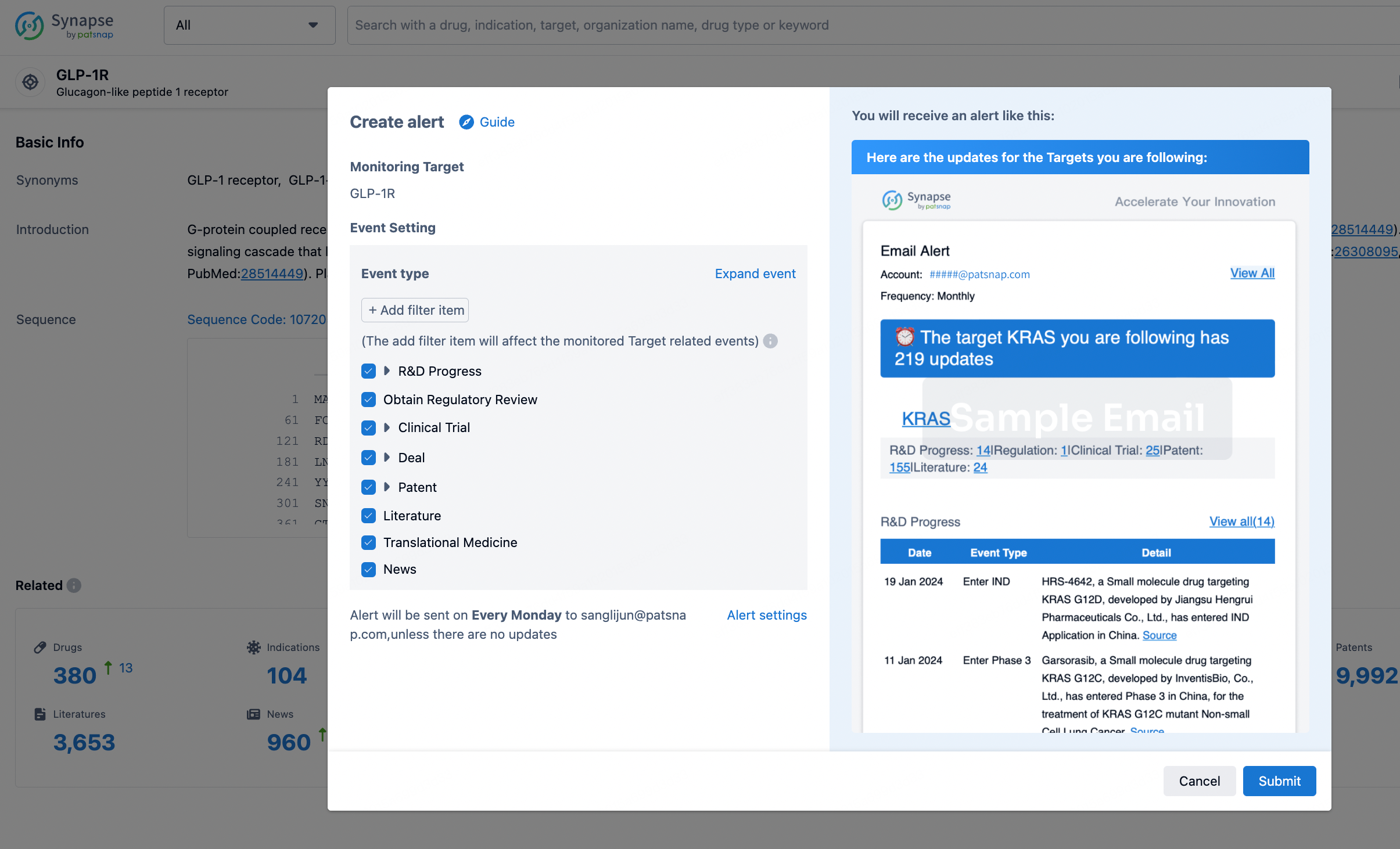
In the Synapse database, you can stay updated on the latest research and development advances of all targets. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!
AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.