A Comprehensive Review of Reslizumab's R&D Innovations and Drug Target Mechanism
Reslizumab's R&D Progress
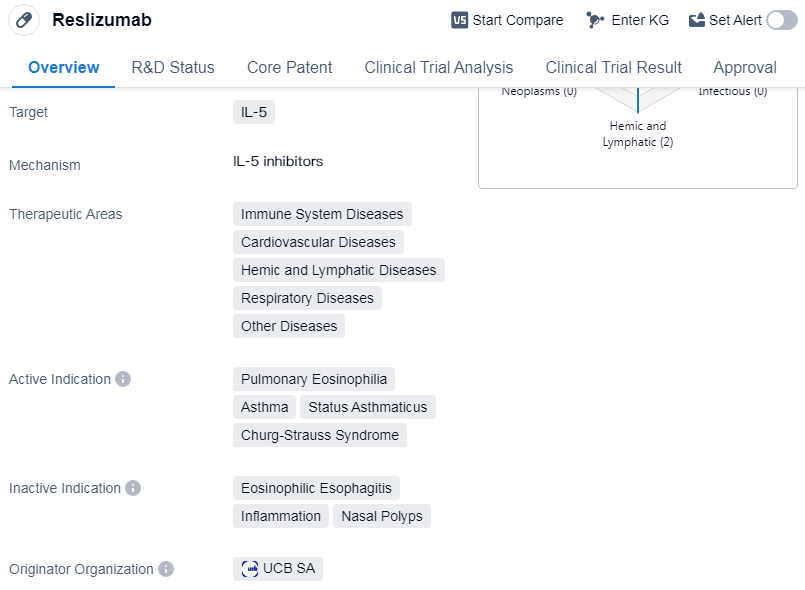
Reslizumab is a monoclonal antibody drug that targets IL-5, a protein involved in immune system regulation. It has been developed to treat a range of diseases related to the immune system, cardiovascular system, hemic and lymphatic system, respiratory system, and other diseases.
The drug has been specifically indicated for the treatment of pulmonary eosinophilia, asthma, status asthmaticus, and Churg-Strauss syndrome. Pulmonary eosinophilia is a condition characterized by an increased number of eosinophils in the lungs, while asthma is a chronic respiratory disease that causes inflammation and narrowing of the airways. Status asthmaticus refers to a severe and prolonged asthma attack that does not respond to standard treatments. Churg-Strauss syndrome is a rare autoimmune disease that affects small and medium-sized blood vessels.
Reslizumab was first approved in the United States in March 2016, making it available for patients in that country. It is important to note that the drug has been classified as an orphan drug, indicating that it is intended to treat rare diseases or conditions. This classification often comes with certain regulatory benefits, such as extended market exclusivity and financial incentives for the drug's development.
The drug's originator organization is UCB SA, a multinational biopharmaceutical company. UCB SA has been responsible for the development and commercialization of Reslizumab. It is worth mentioning that Reslizumab has reached the highest phase of development, which is approval, on a global scale. This indicates that the drug has successfully completed clinical trials and has been deemed safe and effective for its intended use.
However, it is important to note that Reslizumab's highest phase in China is discontinued. This suggests that the drug's development and commercialization in China have been halted.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Reslizumab: IL-5 inhibitors
IL-5 inhibitors are a type of medication that target and inhibit the activity of interleukin-5 (IL-5), a cytokine involved in the immune response. From a biomedical perspective, IL-5 inhibitors are used in the treatment of certain inflammatory conditions, particularly those associated with eosinophilic inflammation.
IL-5 is primarily responsible for the growth, differentiation, and activation of eosinophils, a type of white blood cell involved in allergic and inflammatory responses. By inhibiting IL-5, these inhibitors can help reduce the production and activity of eosinophils, thereby alleviating the symptoms associated with conditions such as severe eosinophilic asthma and eosinophilic esophagitis.
IL-5 inhibitors work by binding to IL-5 or its receptor, preventing the cytokine from interacting with its target cells and exerting its pro-inflammatory effects. This inhibition can help reduce airway inflammation, improve lung function, and decrease the frequency and severity of asthma exacerbations in patients with severe eosinophilic asthma.
Overall, IL-5 inhibitors offer a targeted approach to modulating the immune response and can be effective in managing certain inflammatory conditions characterized by eosinophilic inflammation.
Drug Target R&D Trends for Reslizumab
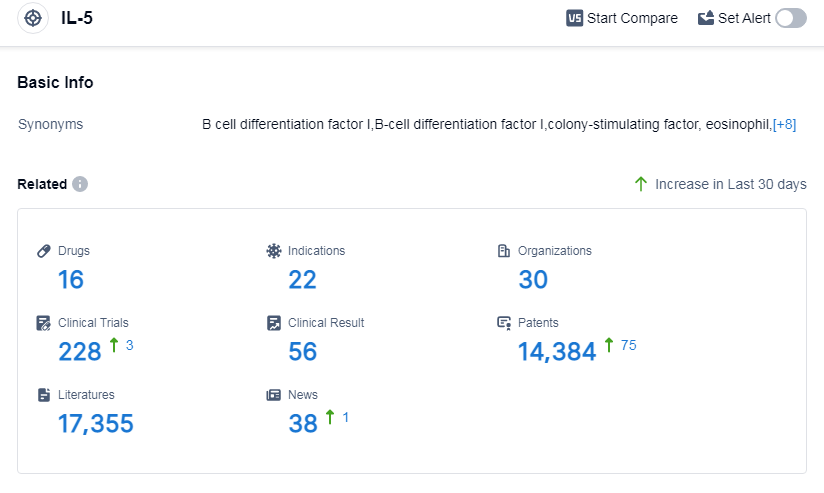
According to Patsnap Synapse, as of 13 Sep 2023, there are a total of 16 IL-5 drugs worldwide, from 30 organizations, covering 22 indications, and conducting 228 clinical trials.
The analysis of the current competitive landscape and future development of target IL-5 reveals that GSK Plc, Teva Pharmaceutical Industries Ltd., and Jiangsu Hengrui Pharmaceutical Group Co. Ltd. are the companies with significant R&D progress. Drugs under the target IL-5 have been approved for various indications, indicating their potential in treating multiple diseases. Monoclonal antibodies, biosimilars, and small molecule drugs are the drug types progressing most rapidly, with intense competition around the innovative drug. The development of target IL-5 is progressing in various countries/locations, including China. Overall, the target IL-5 presents a promising opportunity for pharmaceutical companies and researchers in the future.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Reslizumab is a monoclonal antibody drug that targets IL-5 and has been approved for the treatment of pulmonary eosinophilia, asthma, status asthmaticus, and Churg-Strauss syndrome. The drug's originator organization is UCB SA, it was first approved in the United States in 2016 and is classified as an orphan drug. It's development in China has been discontinued, while its highest phase globally is approval.