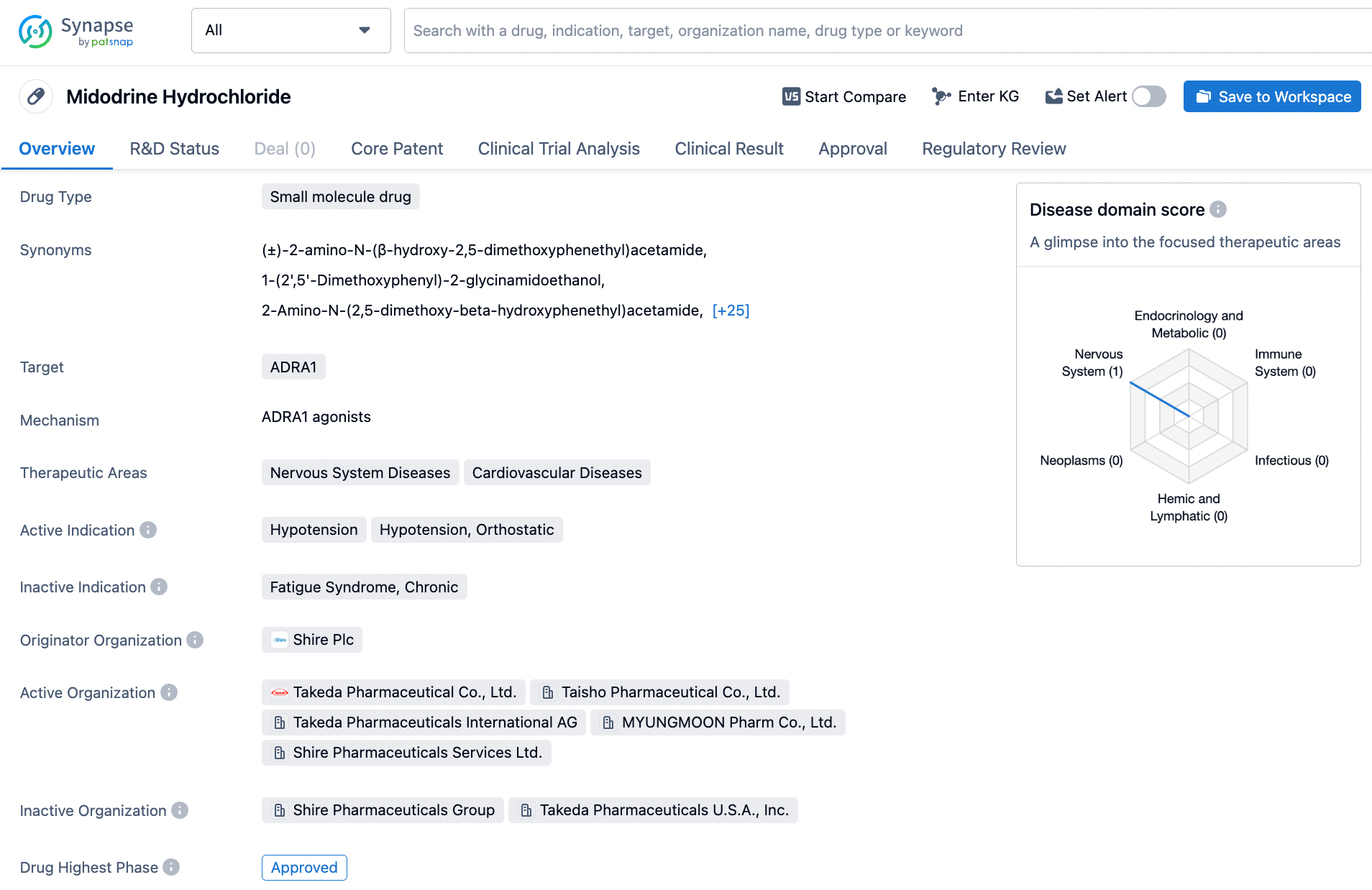
Accessing Midodrine Information on Synapse: A Step-by-Step Approach
Midodrine, a diminutive molecule of therapeutic nature, exhibits its therapeutic effect through its agonistic action upon the α-adrenergic receptor. This pharmacological agent is commonly utilized to ameliorate hypotension, particularly orthostatic hypotension, via its vasoconstrictive actions that lead to a rise in blood pressure. Midodrine obtained approval from the Food and Drug Administration (FDA) in March of the year 1987, and its development can be attributed to the eminent pharmaceutical company, Shire. As an agonist for the α-adrenergic receptor, Midodrine engenders a robust stimulatory response of the sympathetic nervous system, which in turn orchestrates the regulation of both heart rate and blood pressure. By virtue of its multifarious actions, Midodrine has proven efficacy in addressing various maladies stemming from hypotension, encompassing autonomic dysfunction and chronic fatigue syndrome. Click on the image below to begin the exploration journey of Midodrine through the Synapse database!
You can search for the latest pharmaceutical information such as drugs, targets, patents, transactions, clinical results, etc. through the Synapse database. Come and experience it!