Alkermes has started the Vibrance-1 Phase 2 trial to assess ALKS 2680 for treating Narcolepsy Type 1
Alkermes plc has launched the Vibrance-1 study, a phase 2 clinical trial aimed at assessing the safety and effectiveness of ALKS 2680 versus a placebo in individuals diagnosed with type 1 narcolepsy. ALKS 2680, an innovative, experimental oral agent targeting the orexin 2 receptor (OX2R), is being developed by the company for once-daily use in the treatment of narcolepsy, a chronic neurological condition marked by excessive sleepiness during the day.
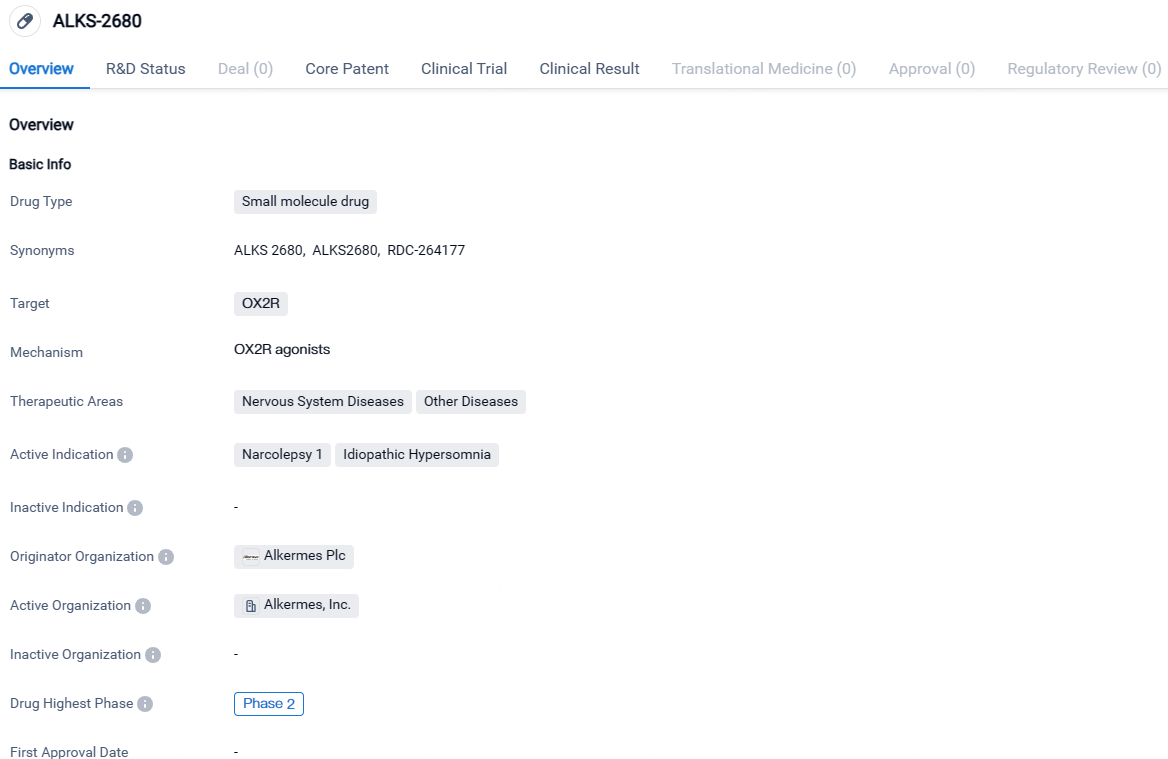
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
ALKS 2680 is being developed to utilize the orexin system, which plays a critical role in maintaining wakefulness, by counteracting the decreased orexin signaling often observed in individuals diagnosed with narcolepsy type 1. "Following encouraging findings from our initial phase 1, proof-of-concept trial, we are pleased to progress this unique oral agent into phase 2," announced Craig Hopkinson, M.D., Chief Medical Officer and Executive Vice President of Research & Development at Alkermes.
Craig Hopkinson further noted, “The commencement of the Vibrance-1 trial represents a key advancement in the progression of ALKS 2680, and we are eager to further explore its safety and effectiveness during this phase 2 trial.”
Vibrance-1 is a phase 2, double-blind, placebo-controlled, randomized, dose-finding study designed to evaluate the effectiveness and safety of ALKS 2680 in individuals with NT1. Participants in the study will randomly receive one of three potential doses or a placebo, administered daily for six weeks.
The primary objective of the study is to determine if those administered ALKS 2680 show a significant reduction in sleepiness relative to those receiving the placebo, measured by the average change in sleep latency using the maintenance of wakefulness test.
Additional objectives of the study include monitoring changes in the Epworth Sleepiness Scale score, the average weekly rate of cataplexy, and the occurrence of adverse events. The study aims to enroll around 80 NT1 patients at locations in the U.S., Australia, and Europe. Those in the double-blind phase of the study will have the opportunity to join a subsequent open-label safety extension phase.
ALKS 2680, a selective orexin 2 receptor agonist and innovative, oral investigational drug, is being developed as a once-daily therapy for narcolepsy. The activation of orexin neuropeptides through OX2R is vital for regulating the sleep-wake cycle, and the degeneration of these orexin-producing neurons in the brain has been linked to excessive daytime sleepiness and cataplexy in those with narcolepsy. ALKS 2680 aims to improve wakefulness duration and control cataplexy by targeting this underlying narcolepsy pathology. The efficacy and safety of once-daily oral dosing of ALKS 2680 were previously evaluated in a phase 1 study involving healthy individuals as well as subjects with narcolepsy type 1, narcolepsy type 2, and idiopathic hypersomnia.
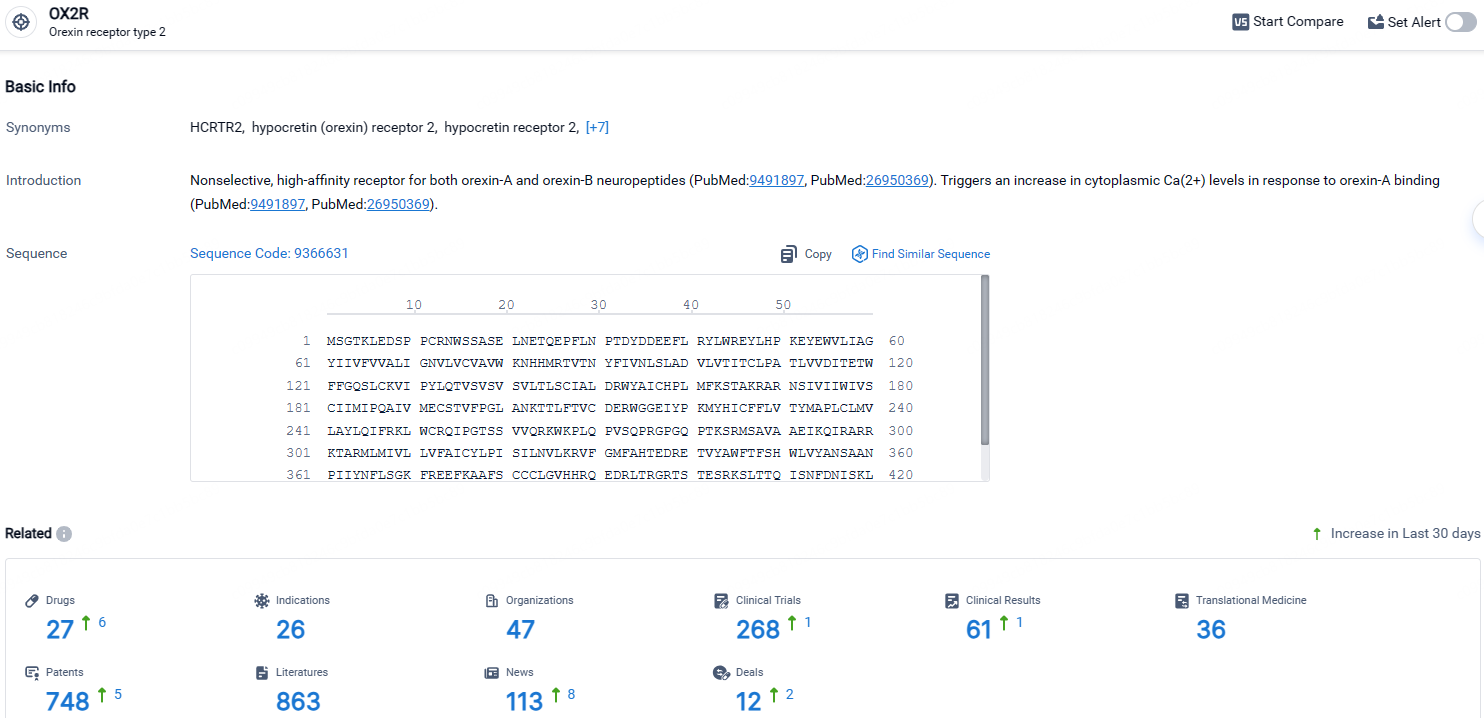
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of April 28, 2024, there are 27 investigational drugs for the OX2R targets, including 36 indications, 47 R&D institutions involved, with related clinical trials reaching 268, and as many as 748 patents.
ALKS-2680 is being developed for the treatment of Narcolepsy 1 and Idiopathic Hypersomnia, both of which are neurological disorders characterized by excessive daytime sleepiness. Currently in Phase 2 of clinical development, ALKS-2680 shows promise in modulating the sleep-wake cycle and potentially providing relief to patients suffering from these conditions.