Almirall Acquires Rights to Novo Nordisk's Anti-IL-21 for Dermatology
Almirall S.A., a worldwide firm specializing in pharmaceutical dermatology, has disclosed a unique licensing arrangement with Novo Nordisk to acquire the exclusive rights for the experimental monoclonal antibody known as NN-8828, which targets IL-21. As part of this deal, Almirall will secure the international rights to both develop and market this innovative monoclonal antibody, specifically for use in managing various dermatological conditions with an immune-inflammatory component.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
 Almirall is poised to expedite the progression of the pharmaceutical candidate, taking on the task of global development and future market release for major skin-related conditions. Novo Nordisk is set to receive an initial payment, with subsequent payments tied to development milestones and sales thresholds, as well as graduated royalties on worldwide sales.
Almirall is poised to expedite the progression of the pharmaceutical candidate, taking on the task of global development and future market release for major skin-related conditions. Novo Nordisk is set to receive an initial payment, with subsequent payments tied to development milestones and sales thresholds, as well as graduated royalties on worldwide sales.
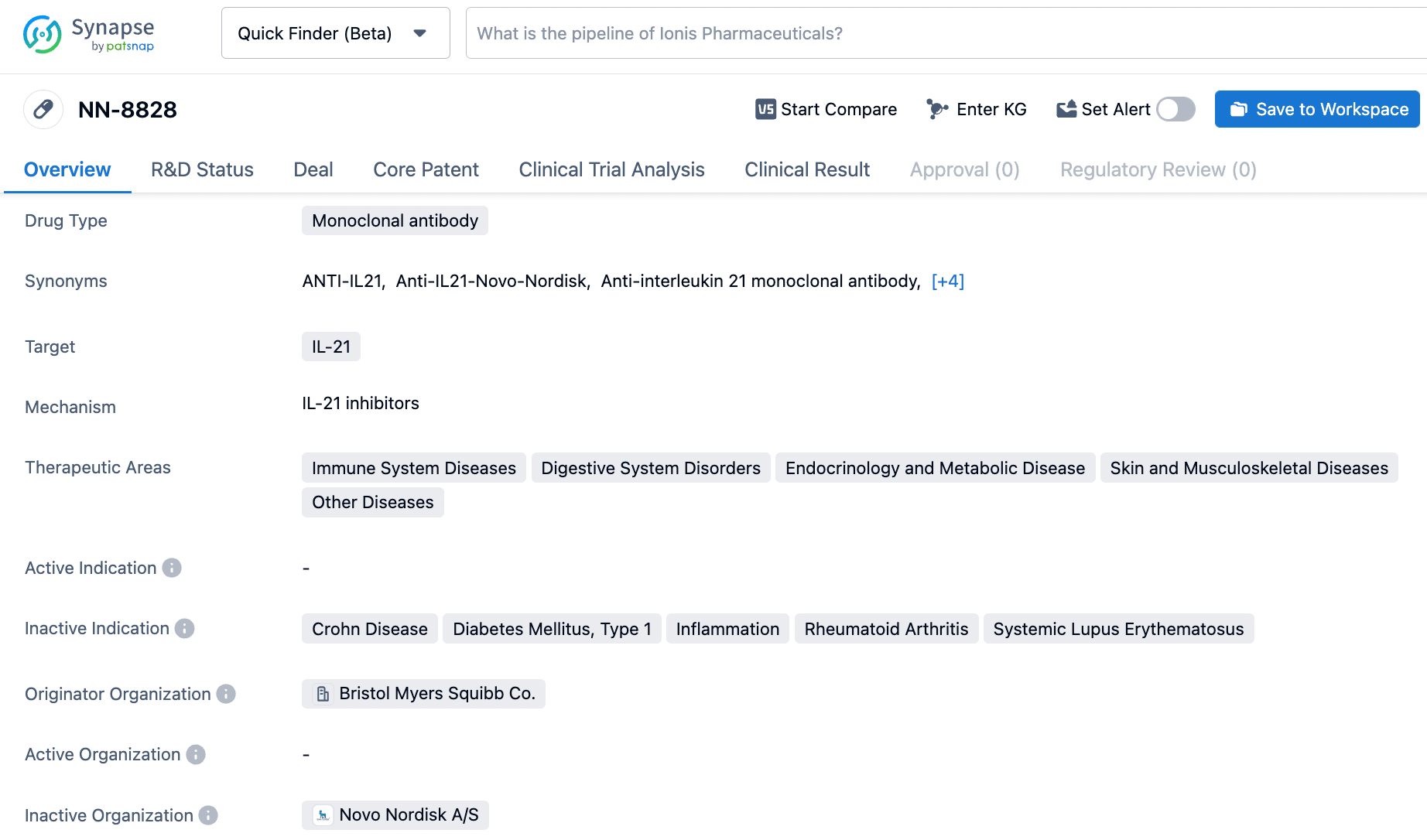
The compound NN-8828 represents a novel class in dermatology, being a high affinity monoclonal antibody focused on the cytokine IL-21. It has advanced to Phase II trials for non-skin related disorders under Novo Nordisk's development efforts.
NN-8828 holds promise in disrupting the downstream signaling cascades of IL-21, potentially curbing the harmful effects this cytokine can have on various immune cells. Its unique action may provide an effective therapeutic approach for treating inflammatory and autoimmune skin conditions.
Dr. Karl Ziegelbauer, Chief Scientific Officer at Almirall, emphasized the company's commitment to advancing therapies for patients with dermatological afflictions. He remarked, "At Almirall, enhancing patient treatment options through innovation is at the forefront of what we do. This collaboration marks a significant step towards utilizing the cutting-edge method of IL-21 inhibition, potentially opening up a novel way to combat a spectrum of skin diseases."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
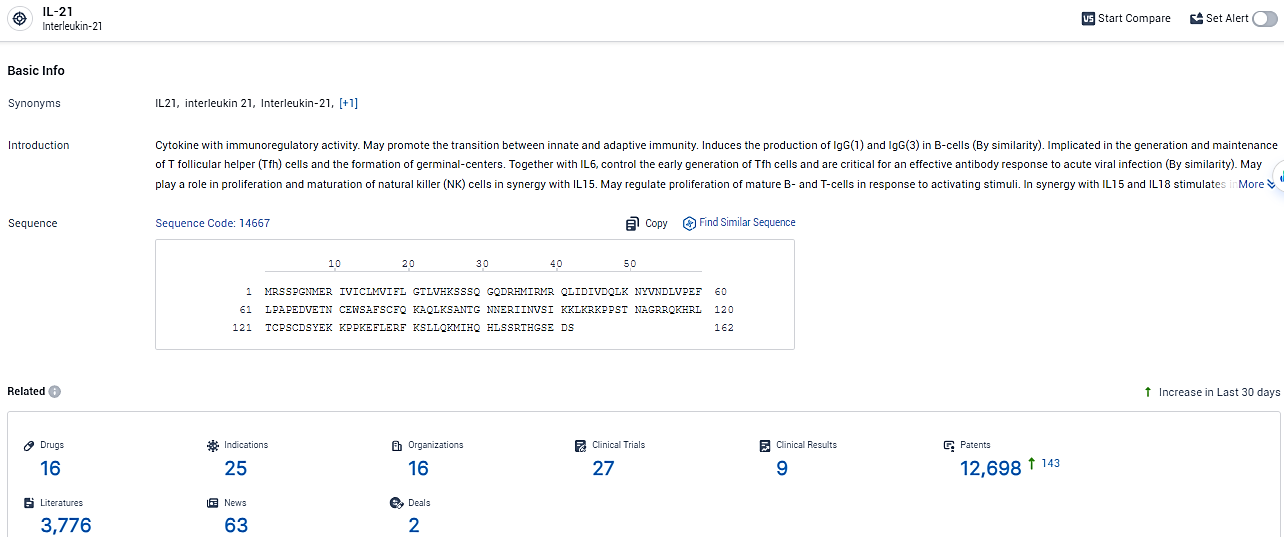
According to the data provided by the Synapse Database, As of February 23, 2024, there are 16 investigational drugs for the IL-21 target, including 25 indications,16 R&D institutions involved, with related clinical trials reaching 27, and as many as 12698 patents.
NN-9828 is a monoclonal antibody that targets IL-21 and is intended to address various therapeutic areas. However, its development has been discontinued, indicating that further research and development of the drug have been halted.