Arrowhead Pharma Reveals Preliminary ARO-CFB Clinical Findings
Arrowhead Pharmaceuticals, Inc. (NASDAQ: ARWR) has revealed preliminary findings from a Phase 1/2a clinical trial of ARO-CFB, their experimental RNA interference (RNAi) therapy that targets complement factor B, which is being assessed as a possible treatment for diseases mediated by complement. The results were shared on December 11, 2024, at the 8th Complement-Based Drug Development Summit taking place in Boston.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Unregulated activation of the complement system can contribute to the advancement of certain kidney diseases, either through a direct pathogenic mechanism or by enhancing the inflammatory and damaging effects of other non-complement disease factors. In a Phase 1/2a clinical trial, ARO-CFB treatment in healthy individuals demonstrated significant and lasting decreases in liver production of complement factor B (CFB), a component involved in the activation of the alternative complement pathway and relevant to the pathogenesis of diseases that involve complement activation. The circulating levels of CFB protein were reduced by an average of up to 90% so far, with additional results at higher dosage levels still to come, and the duration of the response has exceeded 3 months," stated James Hamilton, M.D., MBA, Chief of Discovery and Translational Medicine at Arrowhead. "ARO-CFB also showed marked reductions in indicators of alternative complement pathway activation, with average decreases reaching or nearing 100% in AH50 and Wieslab AP across various dosage levels. These preliminary findings in healthy volunteers give us optimism about the potential of ARO-CFB as we aim to complete Part 1 of the study in the coming months, and then move forward to Part 2, which will involve patients with immunoglobulin A nephropathy, the most prevalent glomerular disease globally."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
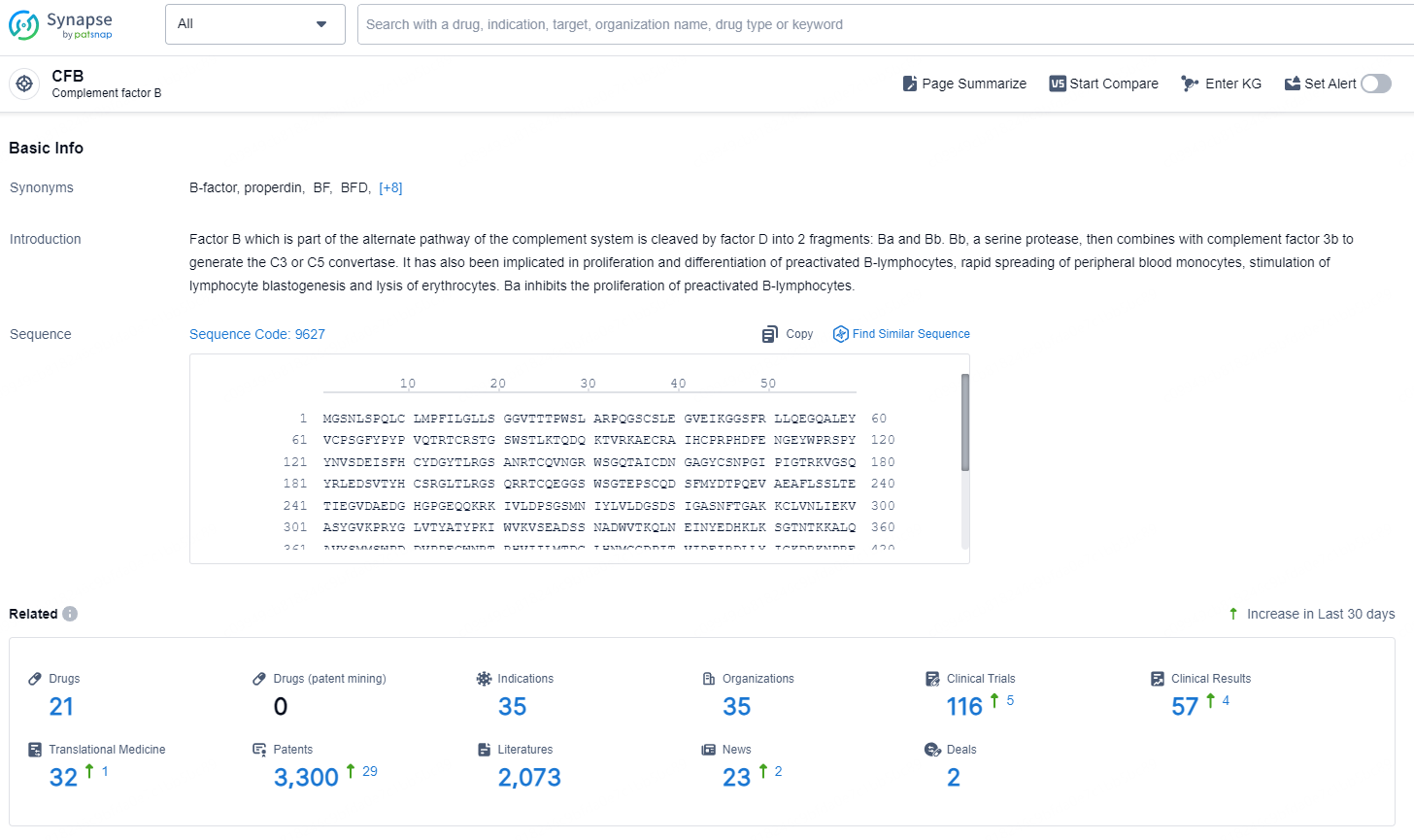
According to the data provided by the Synapse Chemical, As of December 18, 2024, there are 21 investigational drugs for the CFB target, including 35 indications, 35 R&D institutions involved, with related clinical trials reaching 116, and as many as 3300 patents.
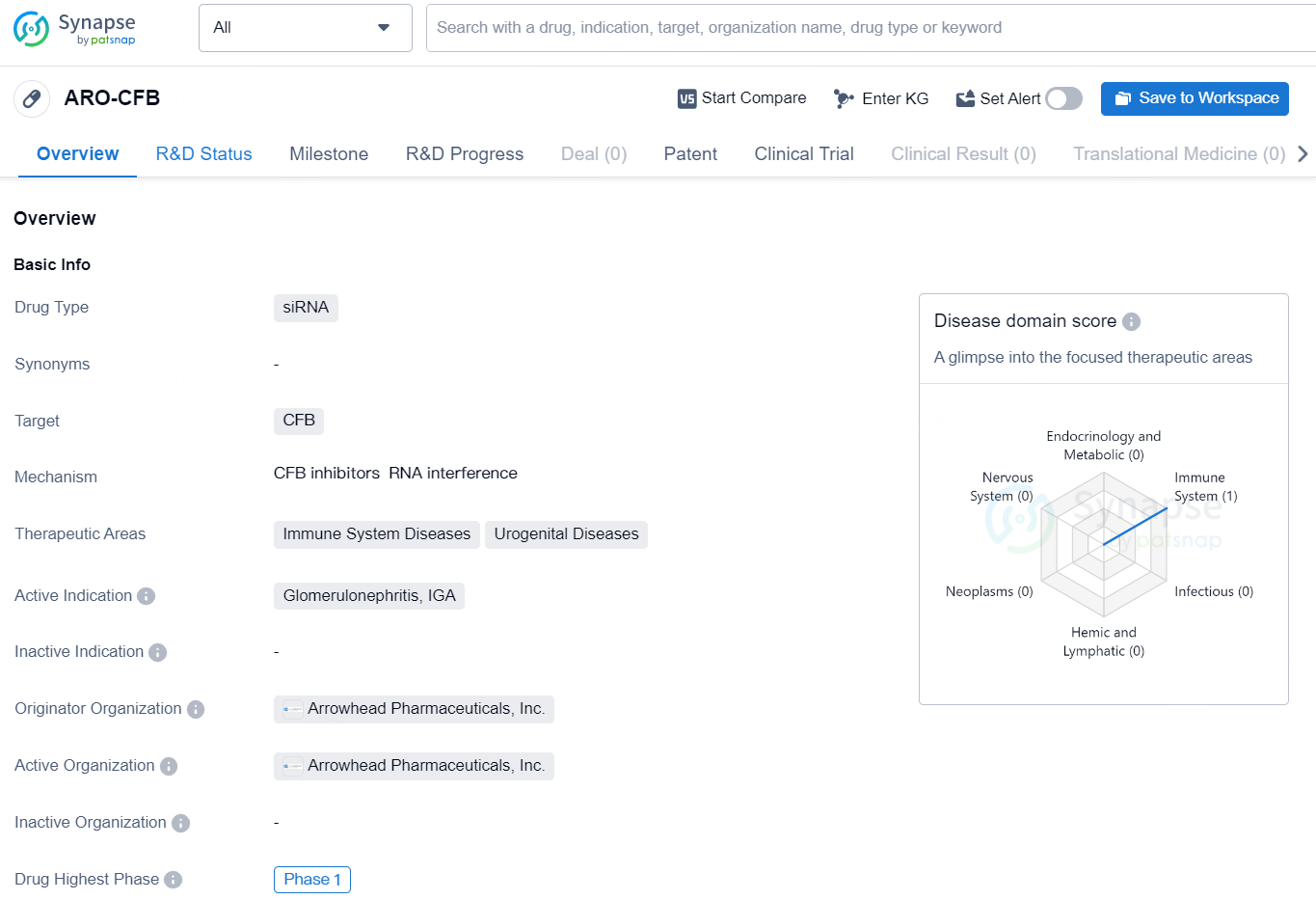
ARO-CFB is a siRNA drug developed by Arrowhead Pharmaceuticals, Inc. The drug targets the complement factor B (CFB) and is intended for the treatment of immune system and urogenital diseases. Its active indication includes glomerulonephritis, IGA. As of the latest available information, ARO-CFB has reached the highest global phase of Phase 1 in its development.