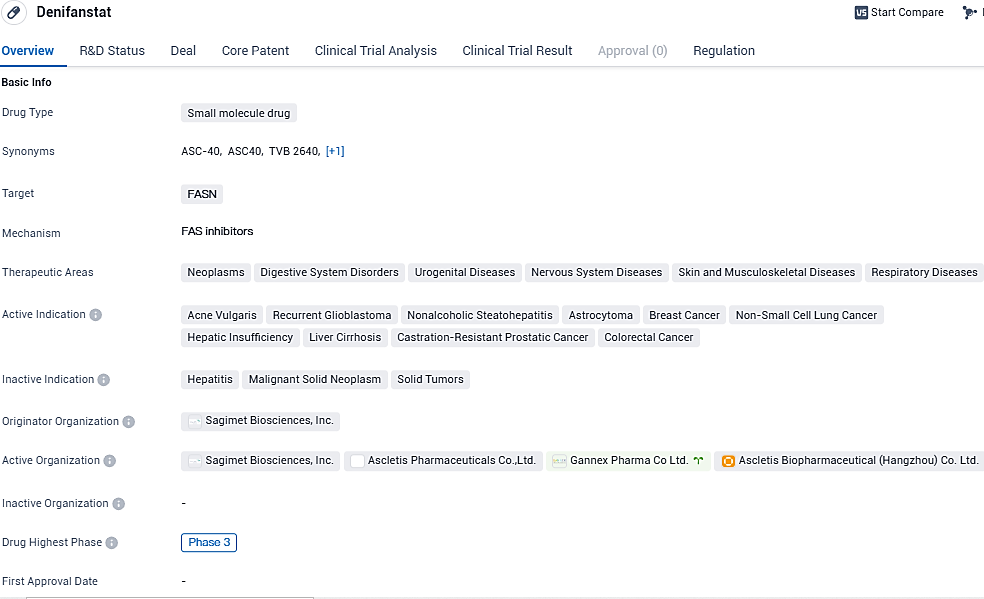
Ascletis has commenced a stage three clinical evaluation for their acne medication, ASC40, also known as Denifanstat
Ascletis Pharma Inc. has declared the commencement of a pivotal Phase III clinical study for its fatty acid synthase inhibitor, ASC40 (Denifanstat) , aimed at addressing the therapeutic needs of individuals with moderate to severe acne vulgaris.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
This third-phase research study is a structured, double-masked, sham-controlled, multi-site examination conducted in China, aiming to assess the safety profiles and effectiveness of ASC40 in individuals with moderate to severe acne vulgaris. It will include 480 participants who suffer from the condition. These individuals will be divided equally, at a 1:1 ratio, between the active therapeutic group and the sham group. Each participant will receive either 50 mg of ASC40 or a corresponding sham orally every day for a duration of 12 weeks.
The main success metrics for the study are based on three critical points: the percentage of participants achieving a defined treatment goal by the 12th week, the change percentage from the starting point in the count of total lesions, and the change percentage from the starting point in the count of inflammatory lesions at the week-12 mark. A successful treatment outcome is characterized by achieving a minimum 2-point decrease in the severity of acne as per the Investigator's Global Assessment score compared to the baseline, alongside achieving a complexion that is either considered clear or nearly clear.
The design of this third-phase trial has received concurrence from the Center for Drug Evaluation of the National Medical Products Administration. Furthermore, it has also been endorsed by the Institutional Review Board at Huashan Hospital, which is affiliated with Fudan University.
On the 2nd of May, 2023, the company Ascletis reported that during its second-phase trial, ASC40 reached its primary and significant secondary goals in the treatment of acne vulgaris. The outcomes indicated a markedly superior therapeutic effect in conjunction with favorable safety data.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
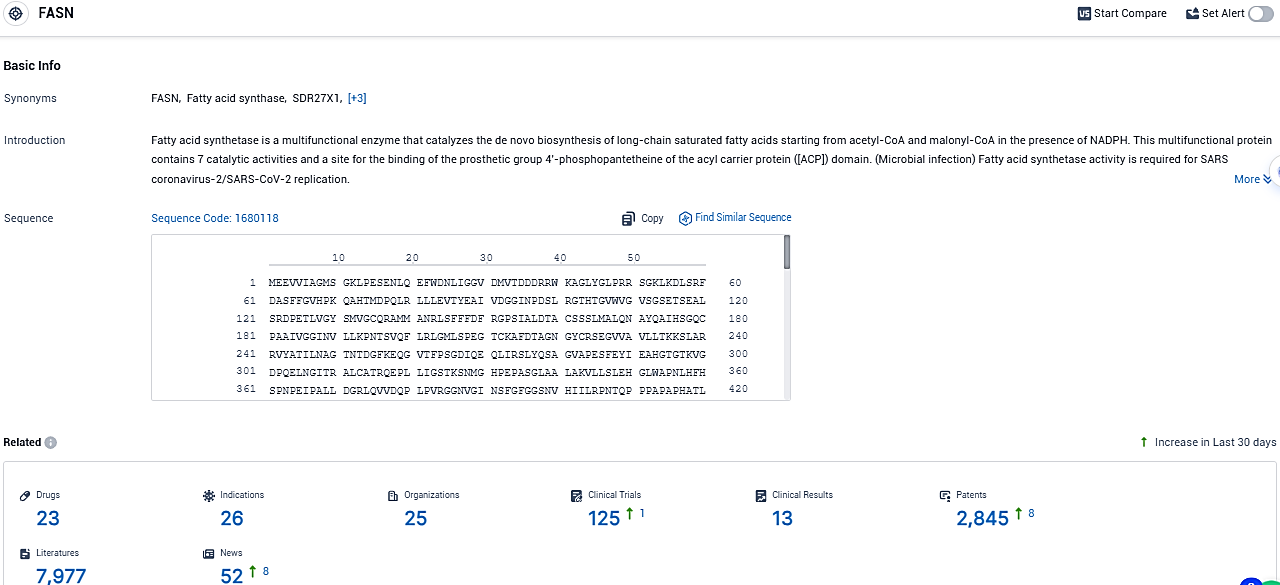
According to the data provided by the Synapse Database, As of December 12, 2023, there are 23 investigational drugs for the FASN target, including 26 indications, 25 R&D institutions involved, with related clinical trials reaching 125, and as many as 2845 patents.
Denifanstat appears to be a promising drug in the field of biomedicine. Its small molecule nature and specific targeting of FASN make it a potentially effective treatment option for various diseases. The fact that it has reached Phase 3 in both global and Chinese clinical trials indicates that it has undergone rigorous testing and has shown promising results in earlier stages of development.