Biogen Acquires HI-Bio for $1.8 Billion to Expand Immunology Portfolio
On May 22, local time, Biogen Inc. and Human Immunology Biosciences (HI-Bio) jointly announced that they had reached an acquisition agreement, in which Biogen agreed to acquire HI-Bio for an upfront payment of $1.15 billion and potential milestone payments of up to $650 million.

HI-Bio is a clinical-stage biotechnology company focused on providing targeted therapies for patients with serious immune-mediated diseases (IMDs). Inspired by the rise of targeted therapies in clinical oncology, the company pursues a treatment strategy that targets and depletes specific types of immune cells driving IMDs. The company's most advanced candidate, felzartamab, is a CD38-targeting antibody that has shown the ability to deplete CD38+ cells, including plasma cells and natural killer (NK) cells, in clinical studies. These cells are implicated in a range of conditions, including antibody-mediated rejection (AMR), IgA nephropathy (IgAN), lupus nephritis (LN), and primary membranous nephropathy (PMN). Felzartamab has received Breakthrough Therapy Designation (BTD) and Orphan Drug Designation (ODD) from the U.S. Food and Drug Administration (FDA) for the treatment of primary membranous nephropathy (PMN). It has also received ODD for the treatment of antibody-mediated rejection (AMR) in kidney transplant recipients.
According to a recent HI-Bio study published in The New England Journal of Medicine, titled "A Randomized Phase 2 Trial of Felzartamab in Antibody-Mediated Rejection," the results of a Phase 2 clinical trial related to felzartamab for antibody-mediated rejection were disclosed.

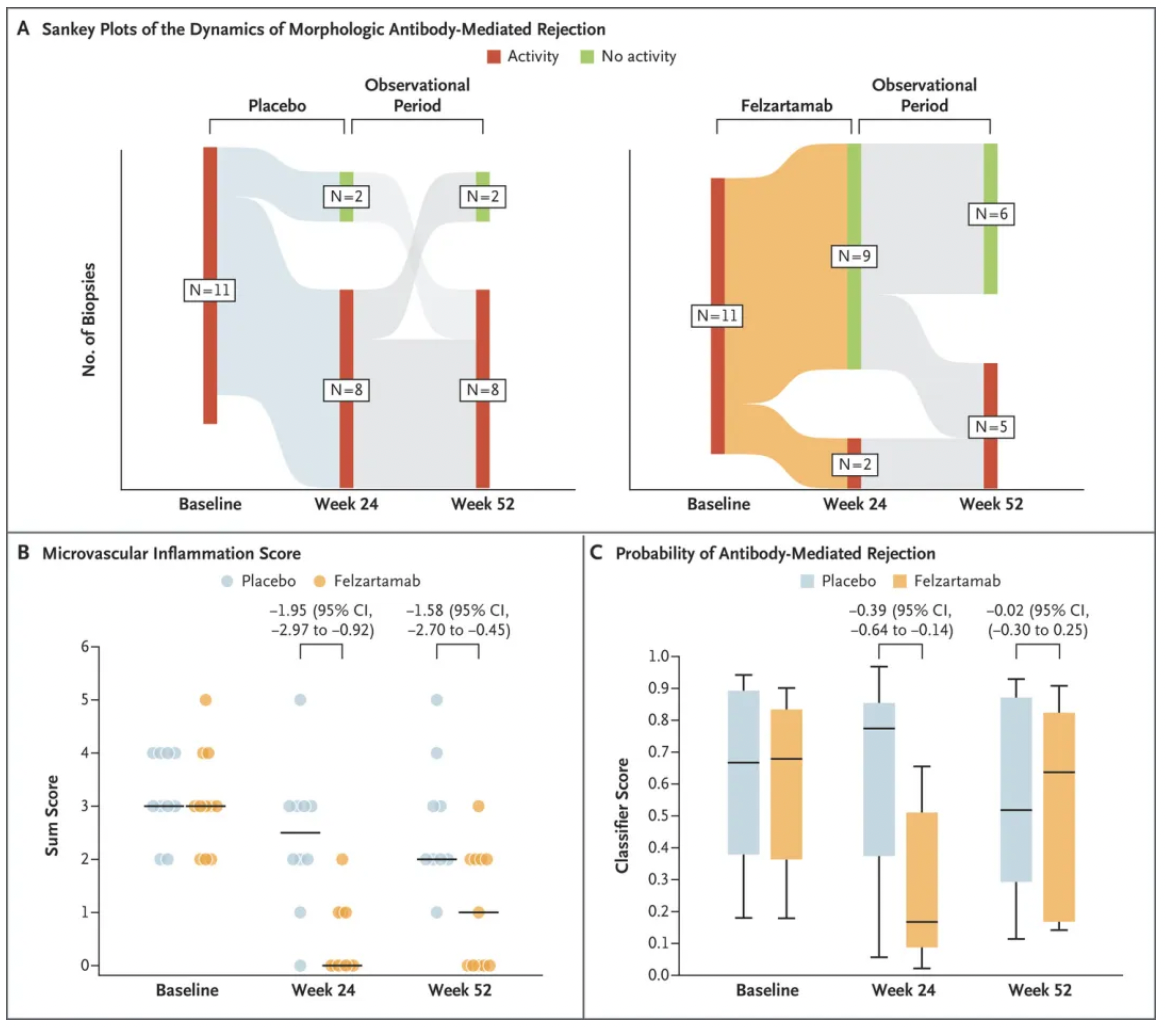
In this phase II, double-blind, randomized, placebo-controlled clinical trial, researchers included patients who experienced antibody-mediated rejection (AMR) at least 180 days post-transplantation. The participants were randomly assigned into two groups: one receiving felzartamab (at a dose of 16 mg/kg body weight) and the other receiving a placebo. The treatment period lasted for 6 months, followed by a 6-month observation period. The primary endpoint was the safety and side effect profile of felzartamab. Key secondary endpoints included renal biopsy results at 24 and 52 weeks, levels of donor-specific antibodies (DSA), counts of peripheral blood NK cells, and levels of donor-derived cell-free DNA.
The results showed that, in the felzartamab group (total of 11 patients), 8 patients experienced mild to moderate infusion reactions. Serious adverse events occurred in 1 patient in the felzartamab group and in 4 patients in the placebo group, with 1 patient in the placebo group experiencing graft loss. After 24 weeks of treatment, the rate of morphological resolution of AMR in the felzartamab group (82%; 9 out of 11 patients) was significantly higher than in the placebo group (20%; 2 out of 10 patients), with a difference of 62 percentage points. The felzartamab group demonstrated lower scores in microvascular inflammation, molecular scores indicative of the probability of AMR, and levels of donor-derived cell-free DNA compared to the placebo group. By week 52, 3 of the 9 patients who initially responded to felzartamab reported a recurrence of AMR, accompanied by a return of molecular activity and biomarker levels to near-baseline levels.
Regarding C5aR1 and the complement system
Notably, in addition to its primary project, felzartamab, HI-Bio's pipeline also includes izastobart/HIB210, a monoclonal antibody product targeting C5aR1 currently undergoing Phase I trials. This product shows potential for further development in a range of complement-mediated diseases, including autoimmune disorders and refractory malignant solid tumors.

The C5aR1 receptor is one of the key receptors in the human complement system. The complement system is a crucial component of the human immune response, primarily responsible for identifying and eliminating pathogens, as well as participating in the clearance of apoptotic cells and the regulation of immune responses. Complement system activation can occur via three pathways: the classical pathway, the alternative pathway, and the lectin pathway. This activation ultimately leads to a cascade of proteolytic reactions, resulting in the formation of the membrane attack complex, which lyses target cells and generates various bioactive fragments. These fragments can bind to specific cell surface receptors, inducing a series of biological effects.
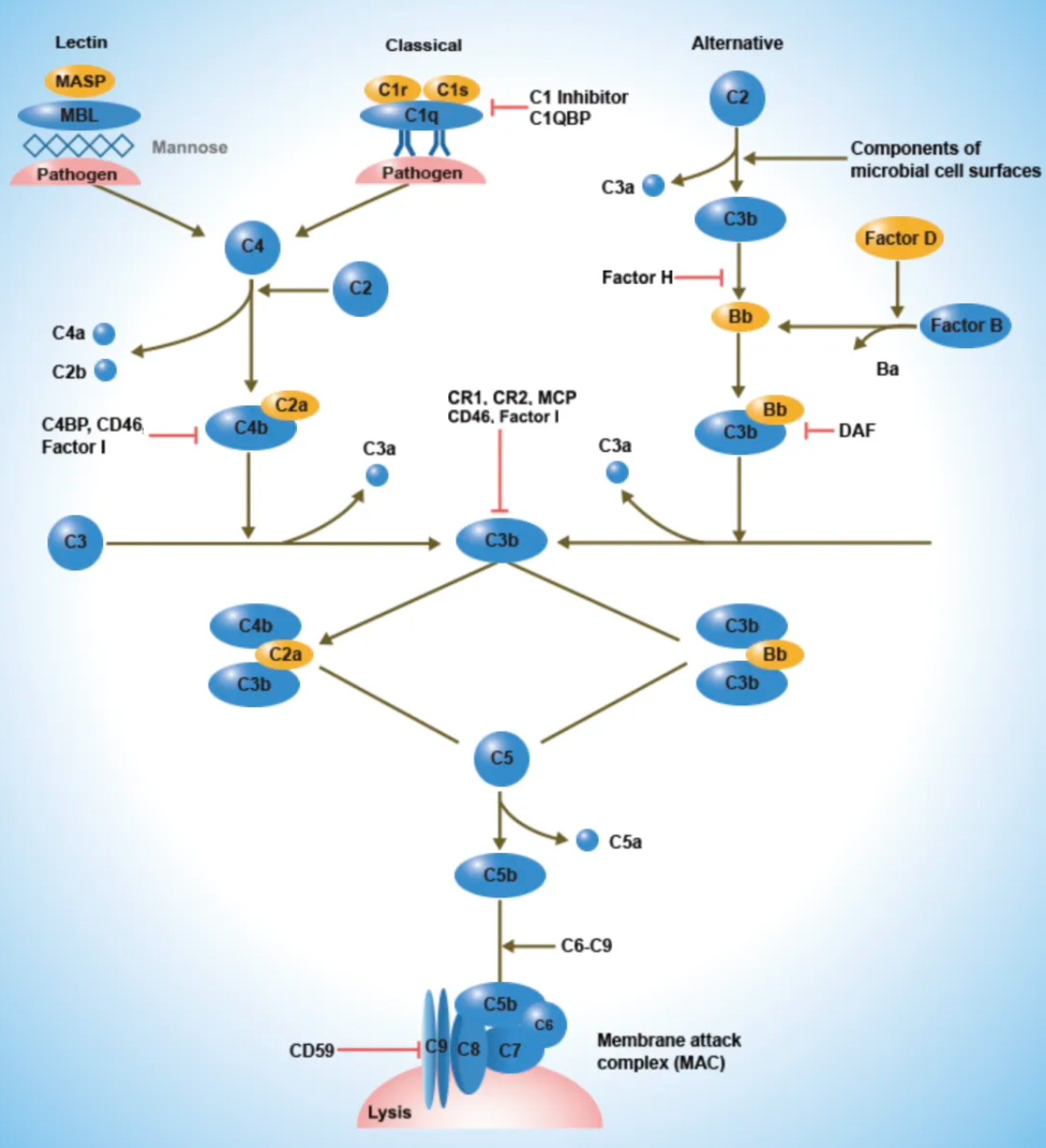
Complement receptors are named according to the complement components they recognize. For example, the receptor that binds to the C3a cleavage product is called the C3a receptor, whereas those binding to the C5a cleavage product are called the C5a receptor 1 (C5aR1) and C5a receptor 2 (C5aR2).
The C5a1 receptor (C5aR1) belongs to the GPCR (G-protein coupled receptor) family and is widely expressed on immune cells such as neutrophils, monocytes, macrophages, dendritic cells, mast cells, and certain T cells, as well as on non-immune cells. Upon binding to C5a, a potent inflammatory fragment generated by the cleavage of C5, the C5a1 receptor robustly activates immune cells, triggering a strong inflammatory response that includes cell activation, migration, depolarization, and oxidative burst. It is a key regulator of complement-mediated inflammatory responses.
The figure below illustrates the wide tissue distribution of the C5a1 receptor in the human body.
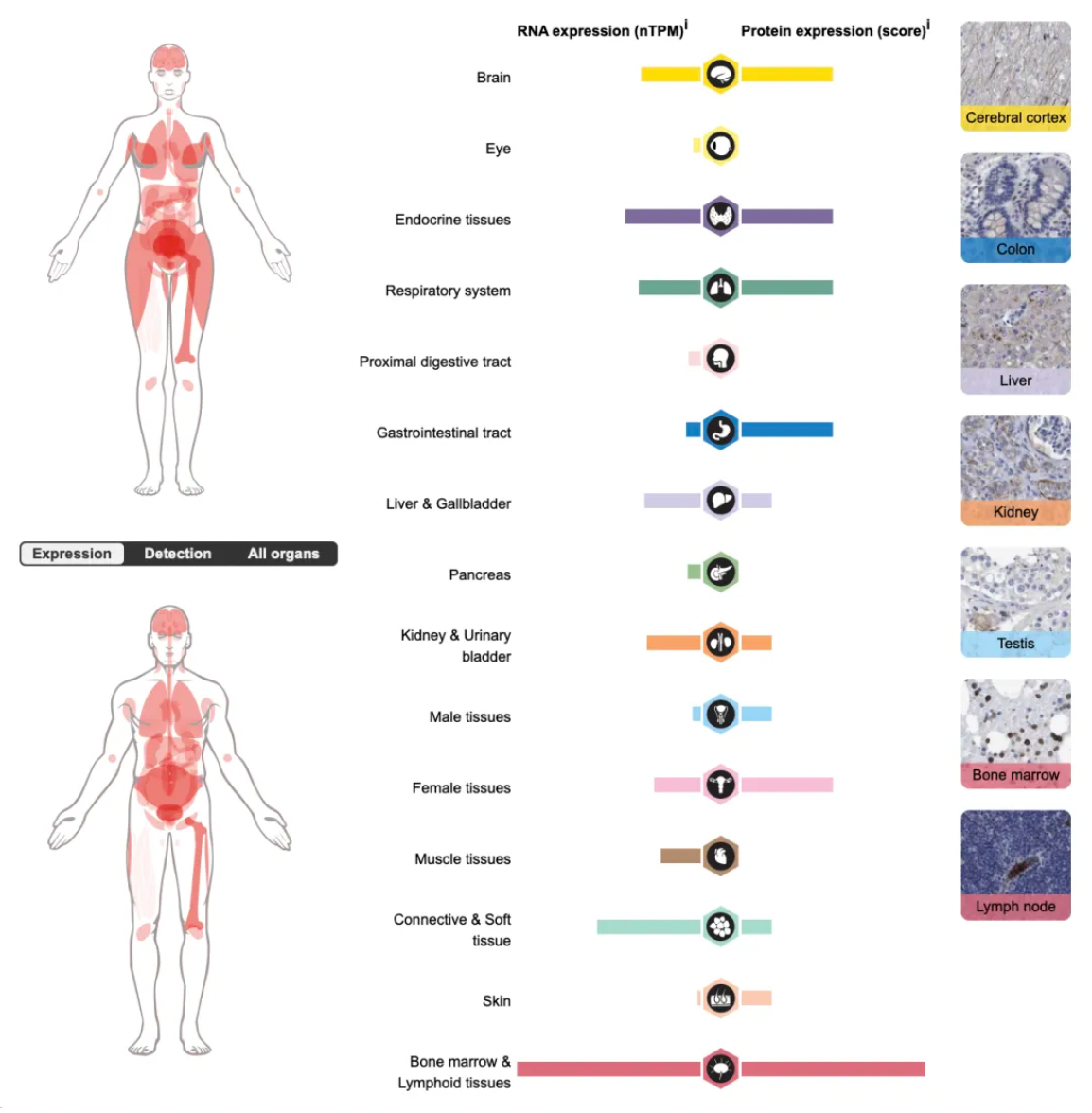
The types of diseases associated with C5a1 receptor dysfunction include cancer, immune system disorders, cardiovascular and metabolic diseases, as well as reproductive and urinary system disorders.
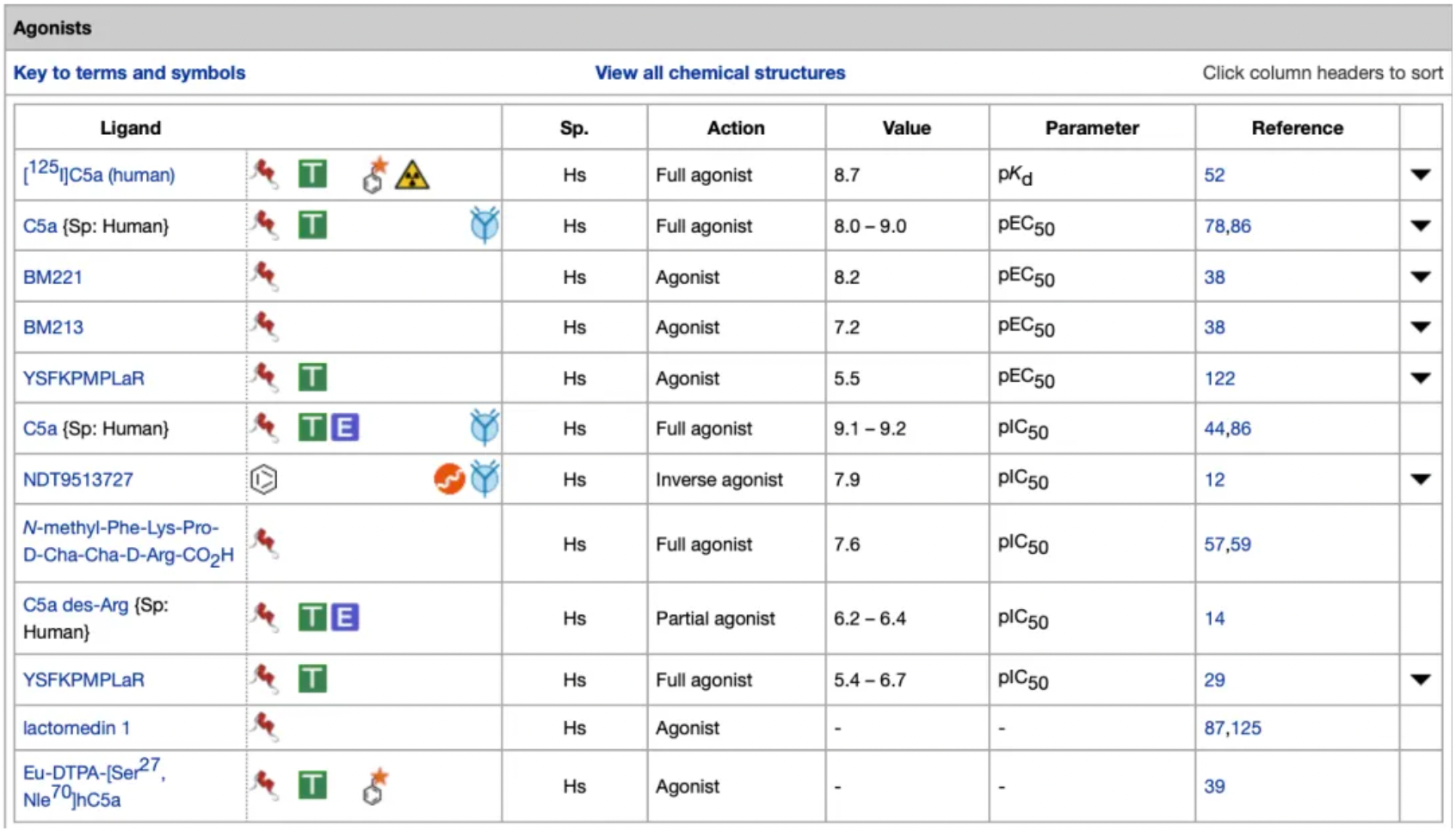
Currently, the major agonist molecules targeting the C5a1 receptor are primarily peptides, with activity ranges distributed between nM and µM.
In contrast, a significant number of small-molecule compounds exist among the antagonists and inverse agonists targeting the C5a1 receptor. Additionally, there are also some peptide-based antagonist molecules.
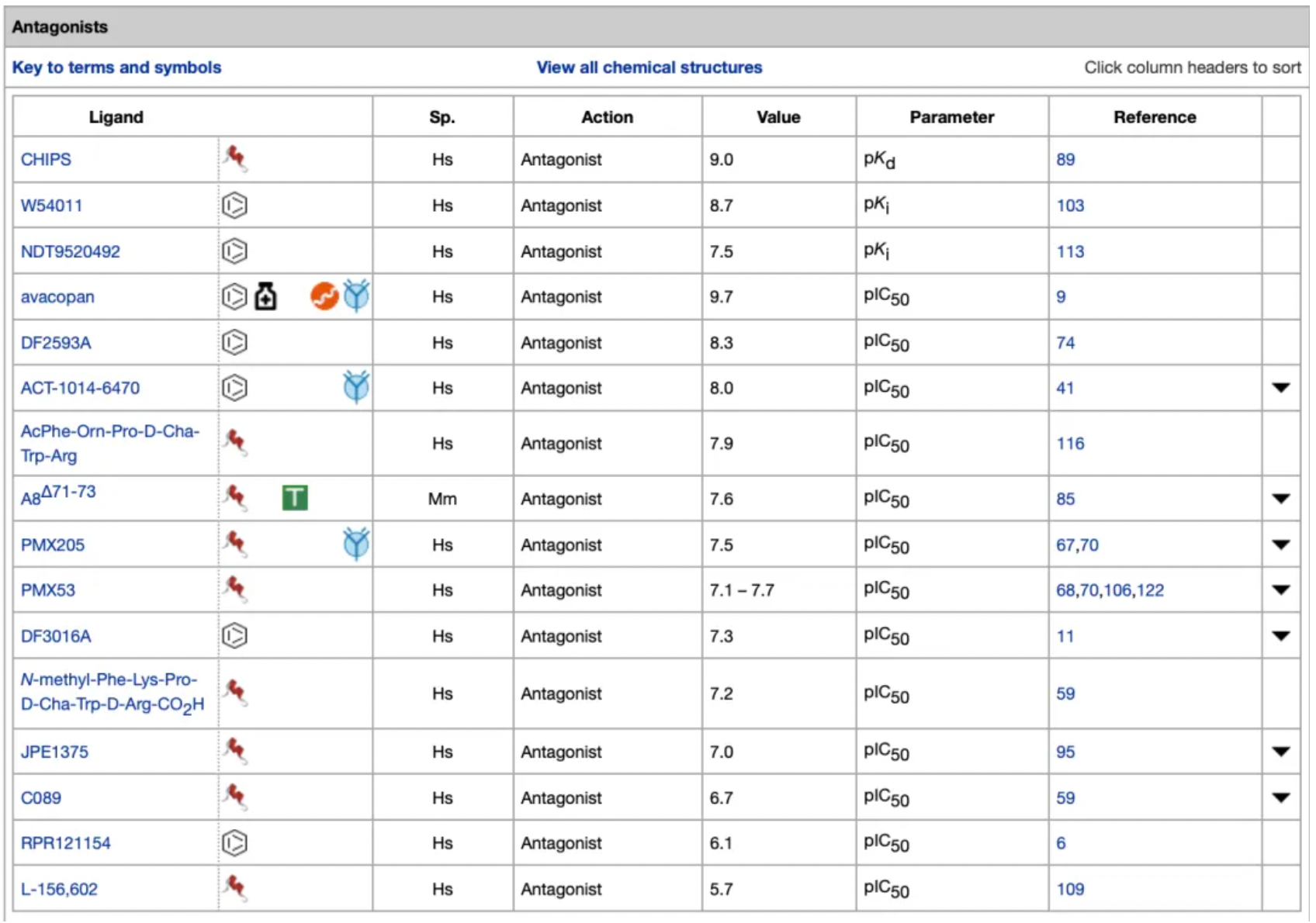
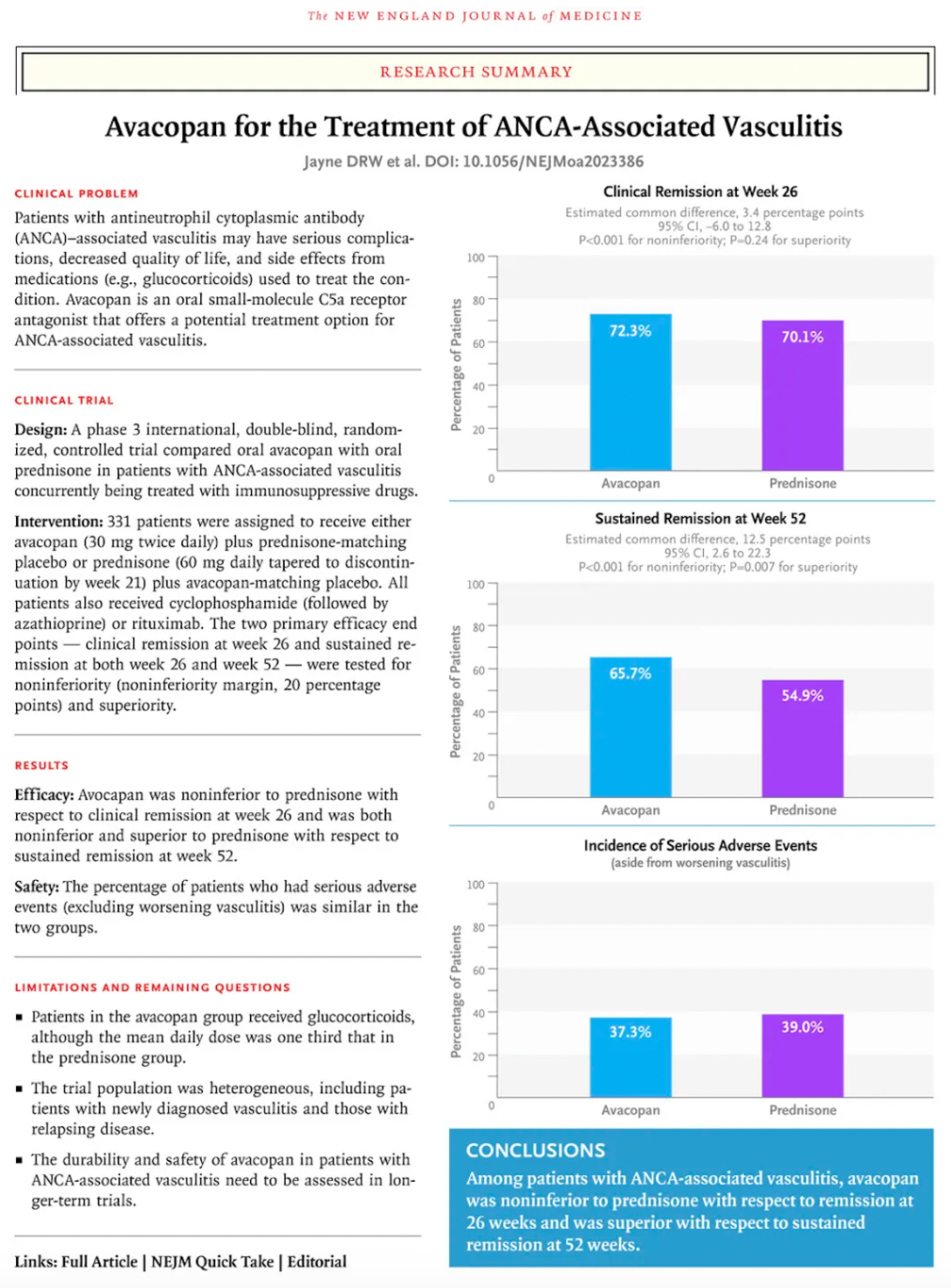
Among them, Avacopan, the molecule with the highest antagonistic activity, was approved for market release in Japan and the United States in September and October 2021, respectively, for the treatment of severe anti-neutrophil cytoplasmic (auto) antibody-associated vasculitis. In January 2022, the European Commission approved Avacopan for use in combination with rituximab or cyclophosphamide for the treatment of adult patients with severe, active granulomatosis with polyangiitis or microscopic polyangiitis.
Data from a pivotal Phase 3 clinical study supporting Avacopan's approval were published in The New England Journal of Medicine.
Another small-molecule antagonist, ACT-1014-6470, developed by Idorsia Pharmaceuticals, has a lower activity compared to Avacopan (pIC50 8.0 vs. 9.7). Two Phase 1 safety studies were published in 2022 and 2023, respectively. Among the 16 study completers, 4 cases of transient and mild adverse events were reported in subjects with severe renal impairment, but 3 of these AEs were deemed unrelated to ACT-1014-6470. The clinical Phase 2 efficacy of ACT-1014-6470 for patients with severe renal impairment remains promising.

Compared to traditional small molecule antagonists, monoclonal antibody drugs targeting C5aR1 are also considered to have significant advantages in inhibiting receptor activity. A representative antagonistic antibody molecule is Avdoralimab (IPH-5401).
Avdoralimab is a humanized monoclonal antibody targeting the C5a receptor 1 (C5aR1) with potential immunomodulatory activity. Upon administration, avdoralimab specifically targets, binds to, and blocks C5aR1 expressed on myeloid-derived suppressor cells and neutrophil subsets. This action prevents its ligand C5a from binding to C5aR1, thereby inhibiting the C5aR1-mediated activation of these cells within the tumor microenvironment. This blockade also curtails these cells' capacity to secrete inflammatory and angiogenic factors. Consequently, this promotes the activation of T cells and natural killer (NK) cells, induces an anti-tumor immune response, and inhibits the proliferation of tumor cells.

Avdoralimab was initially developed by Novo Nordisk. In 2017, the French biotechnology company Innate Pharma acquired the Avdoralimab project from Novo Nordisk for €40 million. Since then, this antibody has been utilized in clinical development for the treatment of solid tumors, COVID-19 infections, and chronic urticaria.
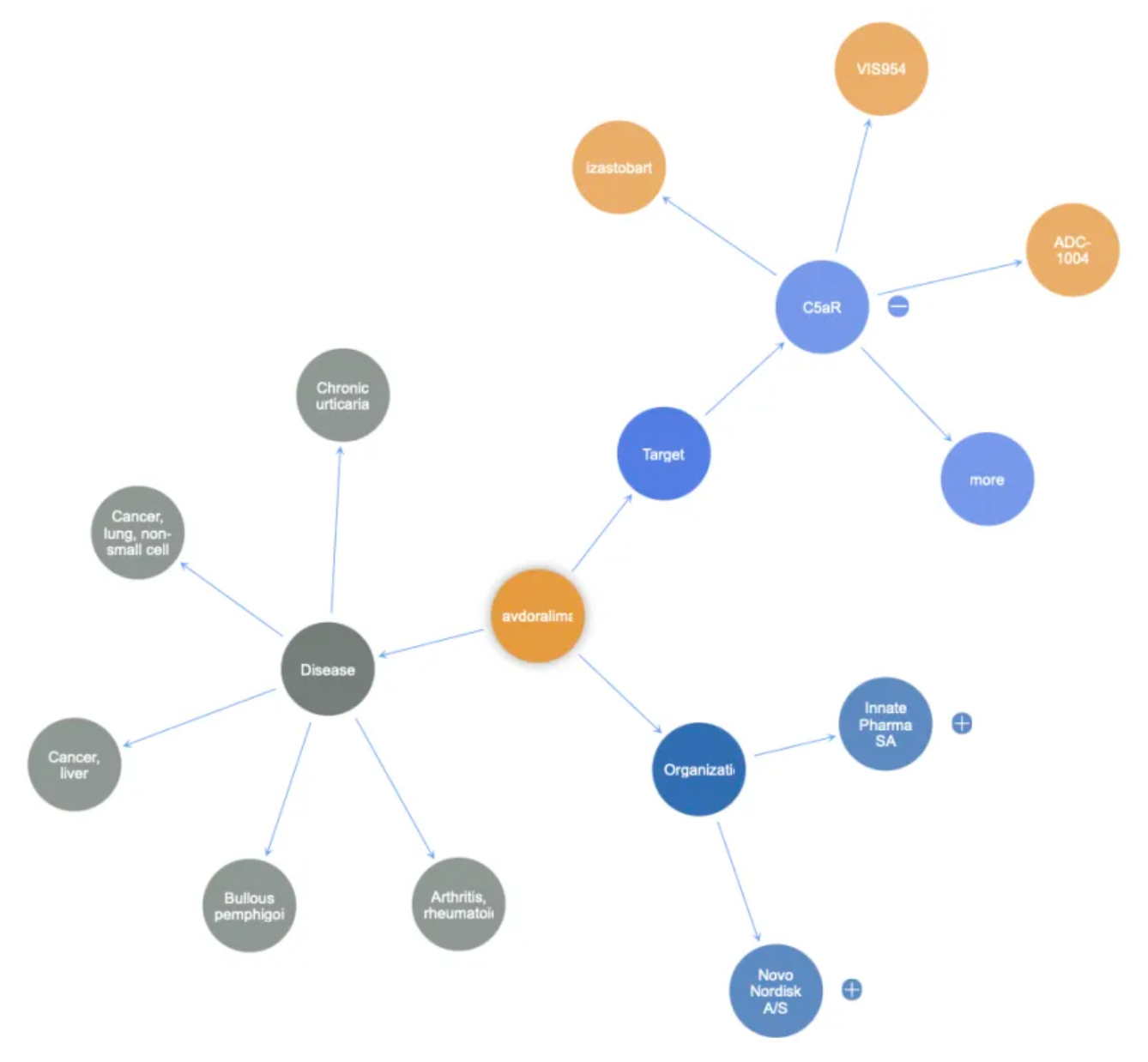
In addition to Avdoralimab, there are several monoclonal antibody products targeting C5aR1 in the market, including Izastobart, originally developed by MorphoSys AG and transferred to HI-Bio, as well as VIS-954, an antagonist monoclonal antibody developed by Visterra.
Izastobart (MOR210) is a humanized antibody that was developed by MorphoSys in the preclinical stage. It targets the C5a receptor and is derived from MorphoSys's HuCAL Platinum® technology. Research indicates that tumors produce significant amounts of C5a, which, by recruiting and activating myeloid-derived suppressor cells (MDSCs), is believed to contribute to the formation of an immunosuppressive pro-tumor microenvironment. MOR210 aims to block the interaction between C5a and its receptor, thereby potentially neutralizing the immunosuppressive function of MDSCs and enabling immune cells to attack the tumor.

In 2018, MorphoSys entered into an exclusive strategic collaboration and regional licensing agreement with I-Mab for MOR210. Under this agreement, I-Mab obtained exclusive rights to develop and commercialize MOR210 in China, Hong Kong, Macau, Taiwan, and South Korea, while MorphoSys retained rights for the rest of the world. MorphoSys received an upfront payment of $3.5 million from I-Mab and is eligible for up to $101.5 million in development and commercialization milestone payments, as well as mid-single-digit tiered royalties on net sales of MOR210 within I-Mab's territories.
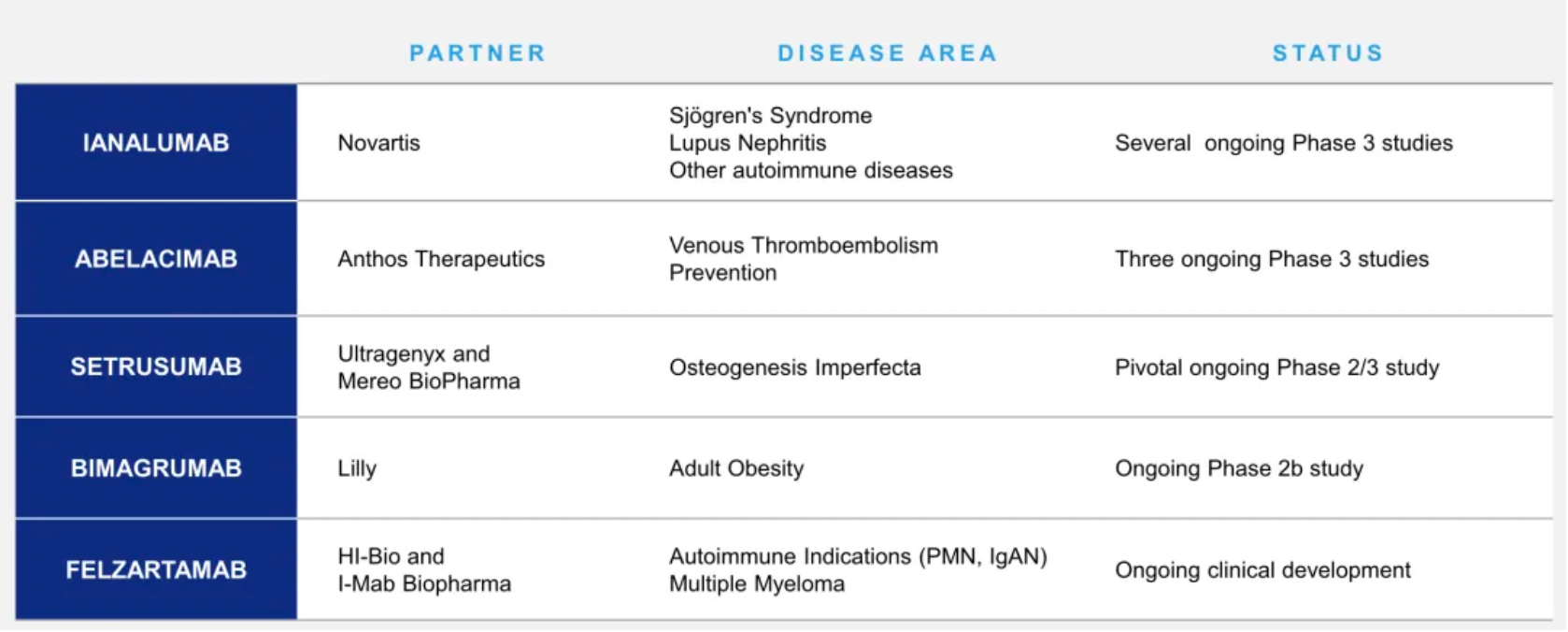
In 2022, MorphoSys transferred the global rights to develop Felzartamab (excluding Greater China) and MOR210 (excluding China and South Korea) to HI-Bio. As part of this agreement, MorphoSys received a 15% equity stake in HI-Bio, along with specific equity earn-out clauses and customary investment rights. MorphoSys also secured a position on the HI-Bio Board of Directors. Upon achieving research, regulatory, and commercialization milestones, MorphoSys is entitled to receive up to $1 billion from HI-Bio for the two projects combined. Additionally, MorphoSys is eligible for low-to-mid double-digit tiered royalties based on net sales of felzartamab and MOR210, along with compensation for ongoing project expenses. HI-Bio will bear all future developmental and commercialization costs. Upon signing, MorphoSys received a $15 million upfront payment for MOR210.
Additionally, MorphoSys has several pipeline products co-developed with Lilly, Anthos, Novartis, and others. On February 5, 2024, MorphoSys and Novartis entered into a merger agreement. Novartis offered MorphoSys shareholders a cash price of €68.00 per share, bringing the total equity value to €2.7 billion. This offer represents a premium of 94% and 142% over the unaffected volume-weighted average prices one month and three months prior to the closing price on January 25, 2024, respectively.
In addition, there is an ADC product targeting the C5a receptor, SAND-5 RGD, currently in the drug discovery phase, developed by Adienne SA.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!