Biohaven Administers First Dose of New Trop-2 Targeting ADC BHV-1510 to Cancer Patient
Biohaven Ltd., an international biopharmaceutical company in the clinical stage, dedicated to finding, developing, and commercializing therapies that can significantly impact a wide variety of both rare and common illnesses, has reported dosing the first patient in a pioneering Phase 1/2 clinical trial for BHV-1510. This innovative Trophoblast Cell Surface Antigen-2 (Trop-2) targeted Antibody Drug Conjugate is the primary ADC initiative to progress into clinical studies within Biohaven’s expanding oncology portfolio.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
 The Phase 1/2 trial of BHV-1510 is a multicenter, open-label investigation targeting patients with specific advanced or metastatic epithelial cell cancers. This trial includes an initial dose-escalation phase, followed by an expansion phase with multiple cohorts.
The Phase 1/2 trial of BHV-1510 is a multicenter, open-label investigation targeting patients with specific advanced or metastatic epithelial cell cancers. This trial includes an initial dose-escalation phase, followed by an expansion phase with multiple cohorts.
Nushmia Khokhar, M.D., the Chief Medical Officer of Oncology at Biohaven, stated, "We are immensely proud to advance our first oncology clinical program with what may be a best-in-class ADC. By starting this monotherapy study, we are advancing towards offering distinctive and superior treatment options to cancer patients. Additionally, we are eager to collaborate with Regeneron to efficiently investigate BHV-1510 in combination with their PD-1 inhibitor Libtayo® across various tumor types."
BHV-1510 is a next-generation, fully optimized Antibody-Drug Conjugate (ADC) that includes a Trop-2 targeting antibody conjugated to a best-in-class Topoisomerase 1 (TopoIx) payload with a uniform drug-antibody ratio of 4. BHV-1510 employs a unique site-specific conjugation technique and highly stable, irreversible linker chemistry developed by GeneQuantum Healthcare Co. (Suzhou) Ltd.
Preclinical studies have shown that BHV-1510 exhibits superior cellular cytotoxicity, bystander effect, and immunogenic cell death, leading to enhanced efficacy as a monotherapy and synergistic efficacy when combined with anti-PD-1 therapy. In studies enabling an Investigational New Drug (IND) application, BHV-1510 also demonstrated a broader therapeutic margin compared to advanced Trop-2 ADCs, including the absence of lung toxicity, potentially translating into a better clinical efficacy and safety profile.
Shiraj Sen, M.D., Ph.D., Director of NEXT Oncology-Dallas, remarked, "Antibody-drug conjugates have shown significant efficacy in solid tumors, but their clinical potential is limited by their safety margins. BHV-1510 provides promising and distinct preclinical data, with the potential for better safety and efficacy across several tumors, particularly those with high unmet need. We are thrilled to partner with the Biohaven team on this significant clinical trial for patients with advanced epithelial cell tumors.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
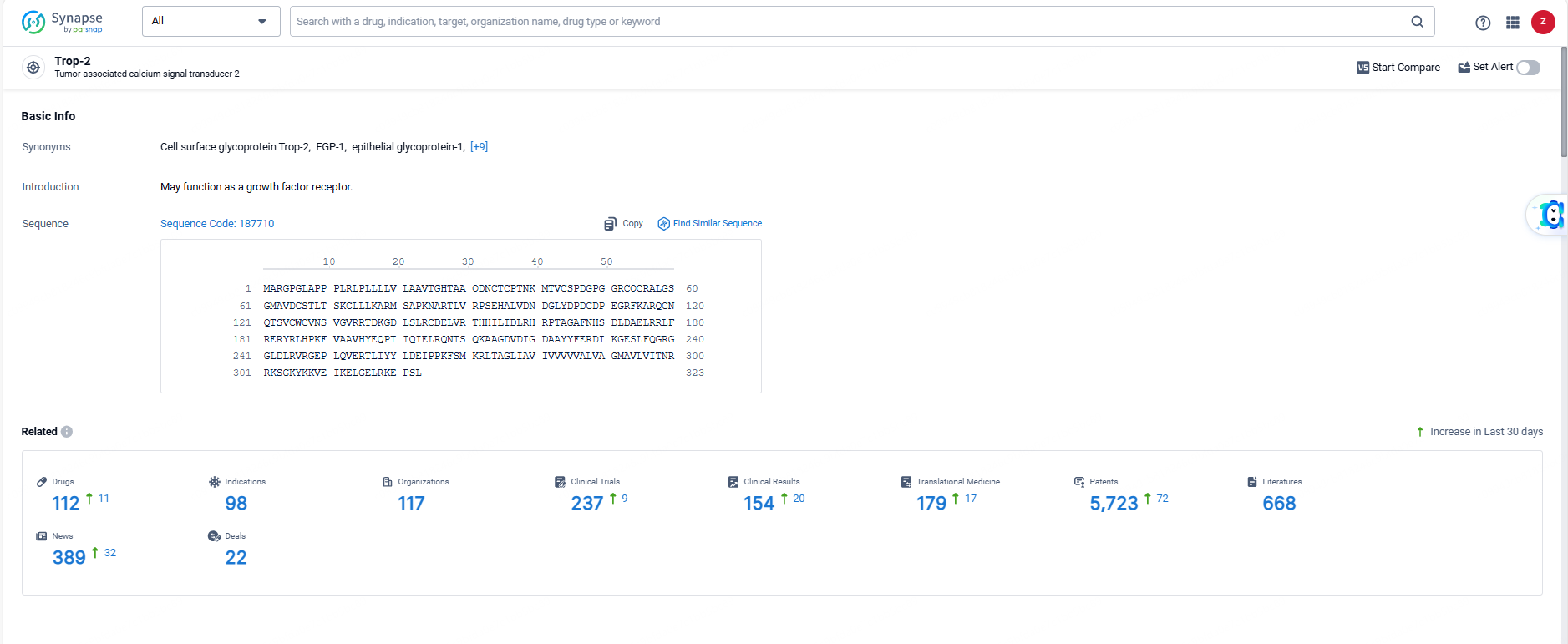
According to the data provided by the Synapse Database, As of June 6, 2024, there are 112 investigational drugs for the Trop-2 target, including 98 indications, 117 R&D institutions involved, with related clinical trials reaching 237, and as many as 5723 patents.
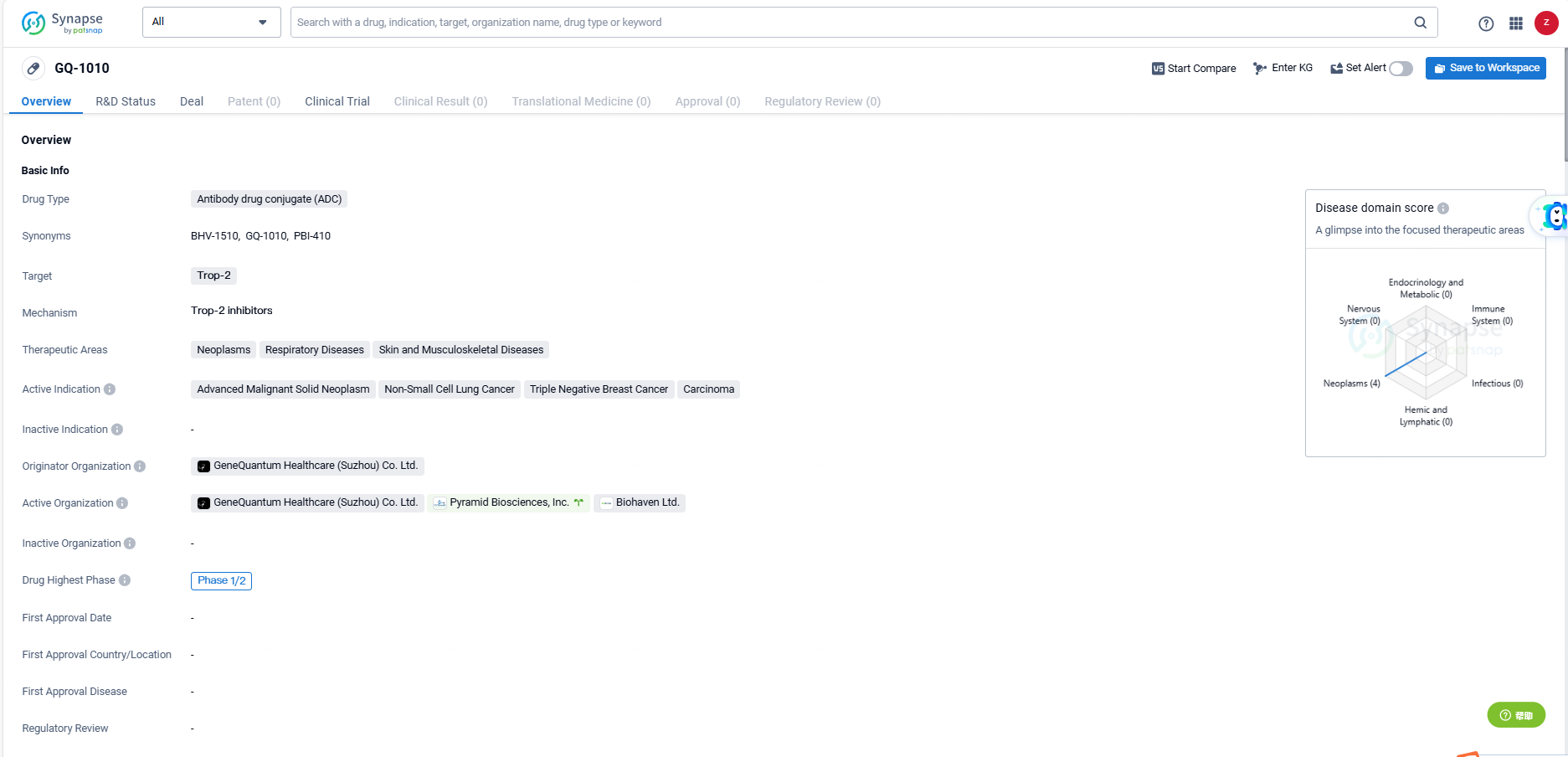
GQ-1010 represents a novel approach to cancer treatment, utilizing the specificity of ADCs to target Trop-2 in various types of cancer. The drug's advancement to later stages of clinical development indicates its potential as a therapeutic option for patients with advanced malignant solid neoplasms, non-small cell lung cancer, triple negative breast cancer, and carcinoma. As the development progresses, further clinical data will be needed to assess the drug's safety and efficacy in these indications.