Biosion and Aclaris Ink Global Licensing Deal for Novel Immunology Products
Biosion, Inc. (Biosion), a biotechnology firm focused on research and development on a global scale, has announced it has secured an exclusive licensing deal with Aclaris Therapeutics (NASDAQ: ACRS). This agreement grants Aclaris worldwide rights (excluding Greater China) to BSI-045B, a potential first-in-class monoclonal antibody targeting TSLP that is currently in clinical development, and BSI-502, a novel bispecific antibody in preclinical development aimed at both TSLP and IL4R, which could potentially be a best-in-class treatment.
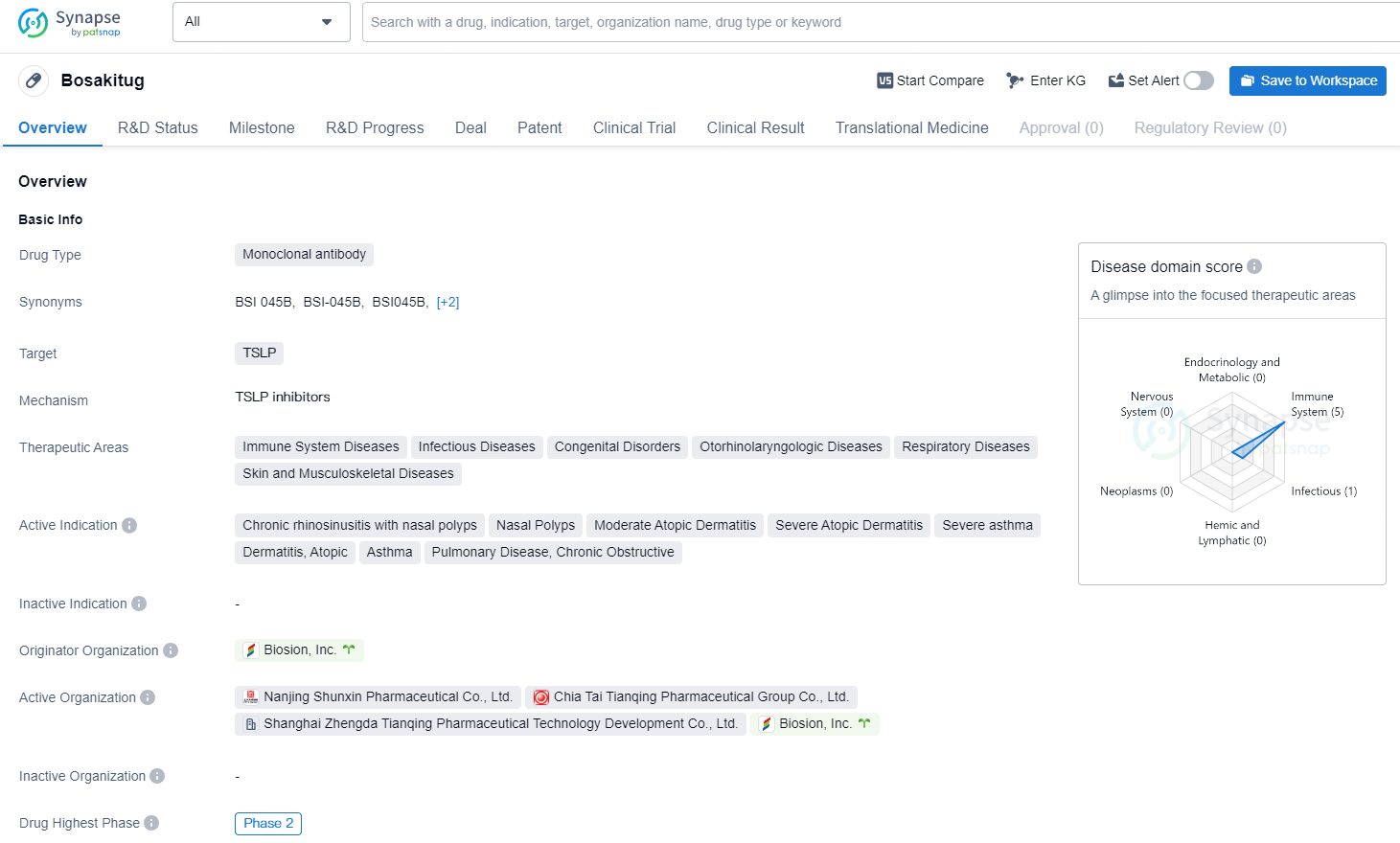
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
According to the agreement, Biosion is set to receive over $40 million in cash as an upfront licensing fee along with reimbursements for specific development expenses and drug product materials. Additionally, Biosion will obtain 19.9% of Aclaris Therapeutics' common stock. The deal also includes regulatory and sales milestone payments that total over $900 million, along with tiered royalties in the low-to-mid single digits based on annual net sales percentage.
“This deal reaffirms the efficacy of our proprietary antibody discovery platforms, as BSI-045B and BSI-502 showcase our ability to develop potential first-in-class and best-in-class drugs,” stated Mingjiu Chen, Ph.D., the Founder and CEO of Biosion. “With the support of our global collaborators and their complementary skills and resources, we aim to deliver more transformative treatments to patients globally.”
“This collaboration represents Biosion’s ninth and tenth global partnered initiatives aligned with our 'discover-development-partnership' approach,” noted Anthony Yeh, Ph.D., Chief Strategy Officer at Biosion. “This alliance will expedite the global clinical development of two pivotal assets and provide the funding needed to propel our other innovative projects toward IND submissions.”
In a completed Phase 2a, single-arm, proof-of-concept trial involving 22 patients with moderate to severe atopic dermatitis in the U.S., BSI-045B exhibited a pharmacodynamic, safety, and efficacy profile that may position it as a leading therapy. Furthermore, BSI-045B is progressing through various Phase 2 trials in China, facilitated by Biosion’s regional partner, Chia Tai Tianqing Pharmaceutical Group, Co., Ltd. (CTTQ), which targets severe asthma and chronic rhinosinusitis with nasal polyps, potentially allowing for proof-of-concept across additional indications.
“This strategic arrangement merges Biosion’s innovative programs with our development strengths in immunology, and we are thrilled to enhance our portfolio with these promising assets,” expressed Dr. Neal Walker, Interim CEO and Chair of the Board of Aclaris. “We are eager to further advance these assets in our mission to provide outstanding therapies for patients globally.”
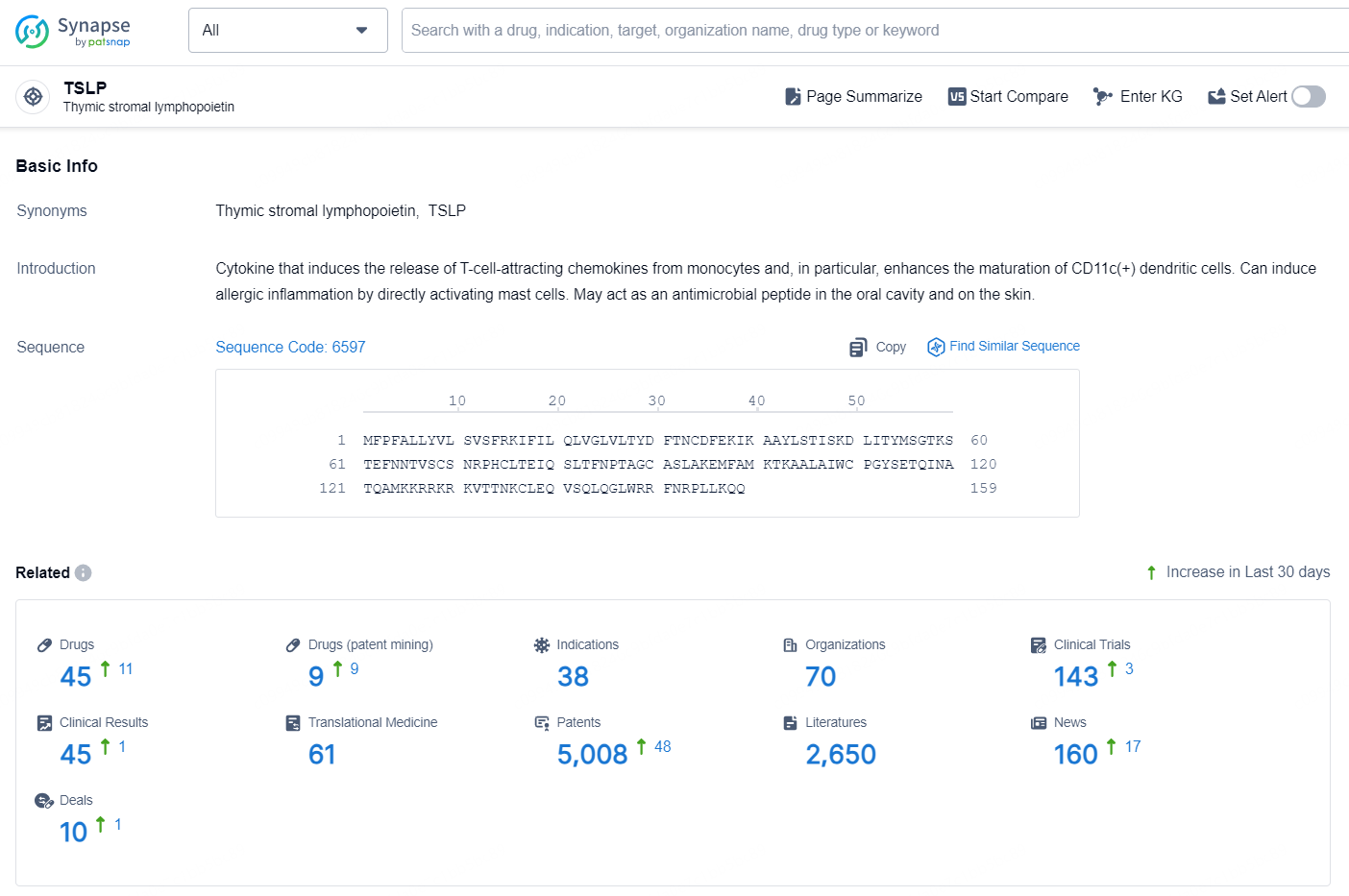
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of November 20, 2024, there are 45 investigational drugs for the TSLP target, including 38 indications, 70 R&D institutions involved, with related clinical trials reaching 143, and as many as 5008 patents.
Bosakitug is a monoclonal antibody drug developed by Biosion, Inc. The drug targets thymic stromal lymphopoietin (TSLP) and is being developed for the treatment of various immune system diseases, infectious diseases, congenital disorders, otorhinolaryngologic diseases, respiratory diseases, as well as skin and musculoskeletal diseases.