Boehringer Ingelheim Advances Promising Geographic Atrophy Therapy Following Positive Phase I Results
Boehringer Ingelheim and CDR-Life have disclosed promising results from the Phase I clinical trial of BI 771716, an investigational antibody fragment designed to preserve vision in individuals with geographic atrophy (GA). As reported in the Study Record on ClinicalTrials.gov, BI 771716 met its primary safety endpoints after being delivered intravitreally in both single and repeated doses. Preparations for the Phase II trial are underway, with the start expected in early 2025.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Geographic Atrophy (GA) represents an advanced, severe stage in dry age-related macular degeneration (AMD), involving progressive retinal degeneration that can lead to irreversible vision loss. This condition significantly contributes to blindness, affecting over five million people worldwide, with more than 40% classified as blind. The vision impairment caused by GA greatly impacts patients' independence, mental health, and quality of life. Current treatment options are notably limited in both effectiveness and availability.
BI 771716, a novel molecule developed by Boehringer Ingelheim in collaboration with CDR-Life technology, is a highly specific antibody fragment intended to optimize penetration across all retinal layers, targeting sites involved in GA pathology. Due to its molecular properties, BI 771716 is promising for achieving exceptional efficacy.
"We are delighted to have achieved this essential milestone in the development of BI 771716 and are now preparing for a Phase II clinical trial to assess its efficacy and dosage," stated Heiko Niessen, Ph.D., Global Therapeutic Area Head for Translational Medicine & Clinical Pharmacology in Retinal Health at Boehringer Ingelheim. "BI 771716 is a key component of our comprehensive retinal portfolio, which underscores our long-term dedication to vision preservation and improving the quality of life for individuals with retinal diseases."
"Achieving this safety milestone is a significant step forward for this compound and highlights our strong collaboration with Boehringer Ingelheim," said Christian Leisner, Ph.D., CEO at CDR-Life. "Having successfully met all four planned milestones up to this point, we are optimistic about the future development of this innovative antibody fragment-based therapy and its potential clinical benefits for geographic atrophy."
"The pursuit of new treatments for geographic atrophy is crucial in the field of age-related macular degeneration," said Charles C. Wykoff, MD, Ph.D., Principal Investigator of the Phase I trial, Director of Research at Retina Consultants of Texas; Chair of Research at Retina Consultants of America; and Deputy Chair of Ophthalmology at the Blanton Eye Institute, Houston Methodist Hospital. "Reaching the Phase I safety milestone is a significant advancement for this promising new treatment for those affected by this vision-threatening condition."
The collaboration and licensing agreement between CDR-Life and Boehringer Ingelheim was announced in May 2020, followed by the selection of an antibody fragment-based therapeutic candidate in September 2021. All milestones have been successfully achieved by the companies to date.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
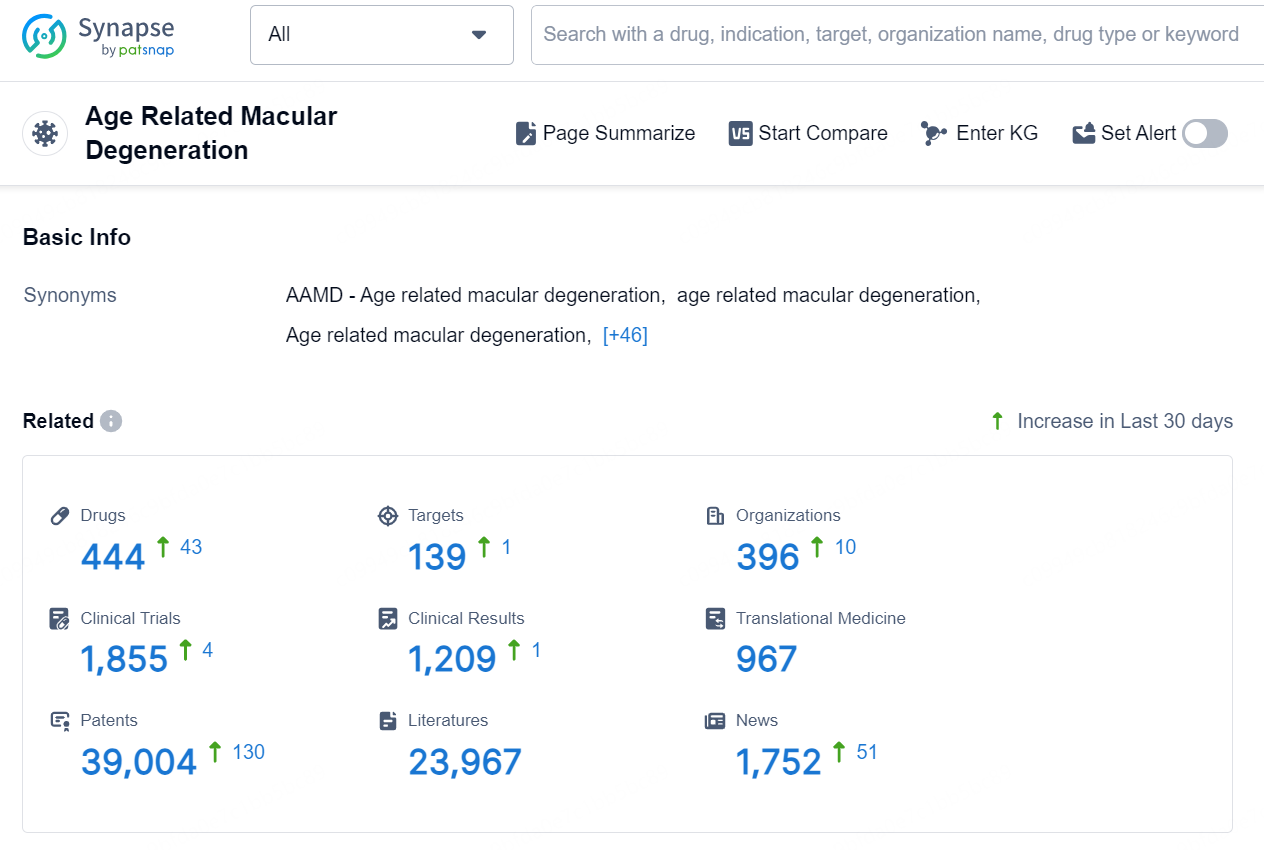
According to the data provided by the Synapse Database, As of September 9, 2024, there are 444 investigational drugs for the Age Related Macular Degeneration, including 139 targets, 396 R&D institutions involved, with related clinical trials reaching 1855, and as many as 39004 patents..
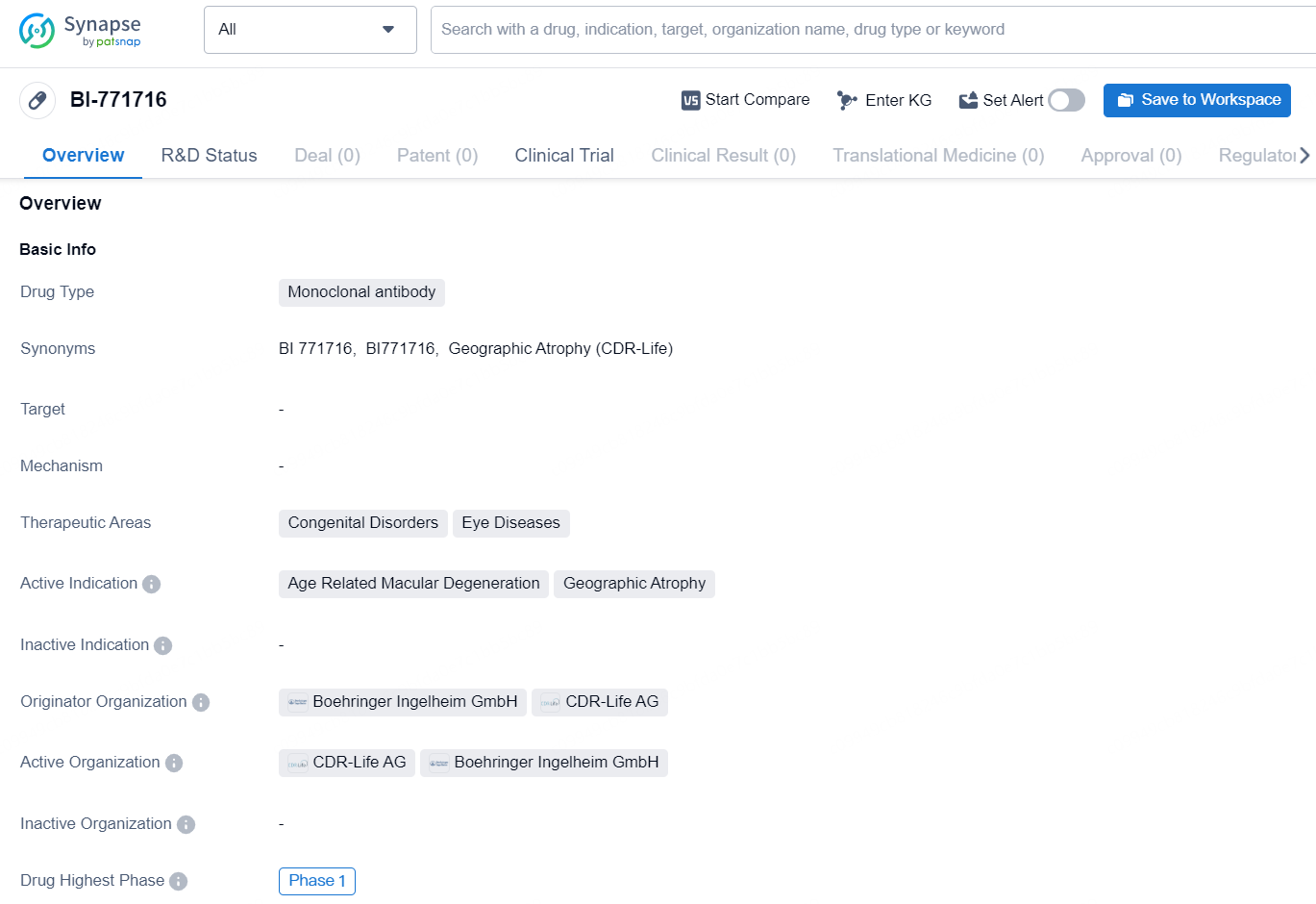
BI-771716 is a monoclonal antibody drug designed to target specific therapeutic areas, including congenital disorders and eye diseases. Its active indications include age-related macular degeneration and geographic atrophy. The drug is in the highest phase of development globally, reaching Phase 1. The originator organizations of BI-771716 are Boehringer Ingelheim GmbH and CDR-Life AG, both of which are involved in the development and production of pharmaceuticals.