Cardiovascular Disease Treatment Drug - PDE3 Inhibitors
Phosphodiesterases (PDEs) are capable of catalyzing the hydrolysis of cyclic nucleotide diester bonds, their family members are numerous, and now we understand there are up to 11 types of PDEs known from mammals - PDE1 to PDE11, and they also exist in different isozymes. The distribution and function of different PDEs also have their own characteristics. Structurally, the protein structure of PDEs can be divided into three parts: the anchoring domain at the N-terminal, the highly conserved catalytic domain at the C-terminal, and the regulatory domain in the middle. Based on the substrates of PDE hydrolysis, PDEs can be divided into three categories: the first type specifically hydrolyzes cyclic adenosine monophosphate (cAMP), including PDE4, PDE7, and PDE8; the second type specifically hydrolyzes cyclic guanosine monophosphate (cGMP), including PDE5, PDE6, and PDE9; the third type can hydrolyze both cAMP and cGMP, including PDE1, PDE2, PDE3, PDE10, and PDE11.
The main therapeutic targets of cardiovascular diseases are mainly focused on PDE3 and PDE5, which have prominent effects in the cardiovascular system. PDE3 is distributed in the heart, vascular smooth muscle, platelets, kidneys, liver, immune cells, etc.; its functions include regulating myocardial contractility, platelet aggregation, vascular smooth muscle contraction, renin release, and insulin signal transduction. PDE3 has the ability to hydrolyze both cAMP and cGMP, but its ability to hydrolyze cAMP is about ten times that of cGMP.
PDE3 Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 22 Sep 2023, there are a total of 68 PDE3 drugs worldwide, from 81 organizations, covering 72 indications, and conducting 478 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.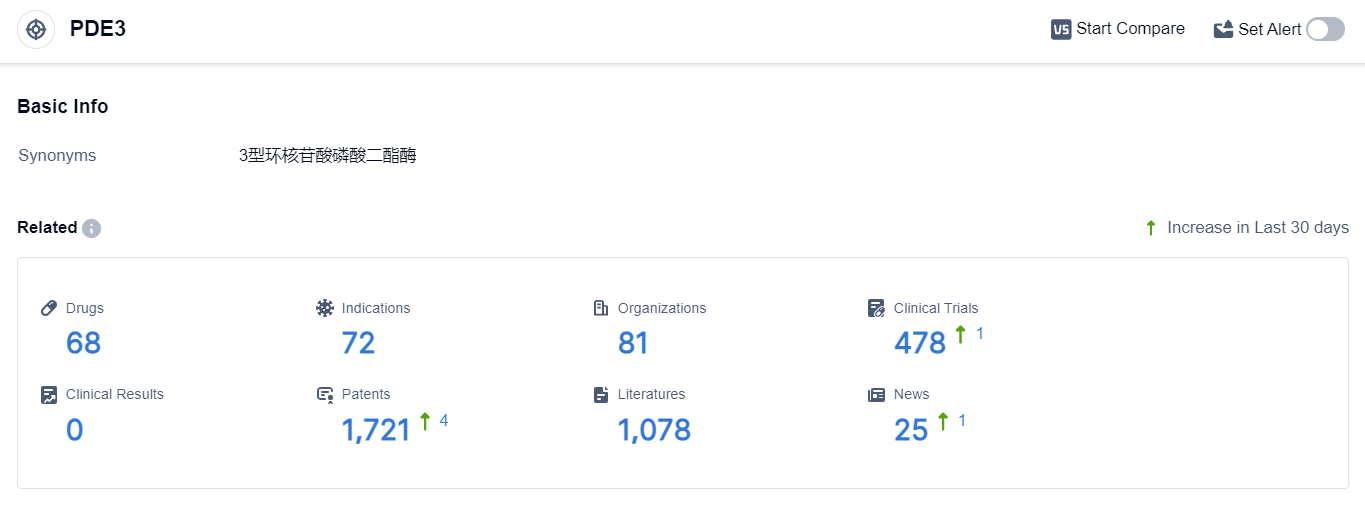
The current competitive landscape of target PDE3 is characterized by the presence of multiple companies actively involved in research and development activities. Companies such as Shandong Lufa Pharmaceutical Investment Co., Ltd., Otsuka Holdings Co., Ltd., Takeda Pharmaceutical Co., Ltd., Orion Oyj, SK Chemicals Co. Ltd., KYORIN Pharmaceutical Co., Ltd., Eisai Co., Ltd., Viatris Inc., Shenzhen Neptunus Pharmaceutical Co. Ltd., AbbVie, Inc., CASI Pharmaceuticals, Inc., He Bei Yi Pin Pharmaceutical Co Ltd, Verona Pharma Plc, MediciNova, Inc., Tenax Therapeutics, Inc., Laboratory Corp. of America Holdings, Yooyoung Pharm Co. Ltd., Nuance Pharma (Shanghai) Co., Ltd., Medpace Holdings, Inc., and Kowa Co., Ltd. are leading the development of drugs targeting PDE3. These companies have multiple drugs in different phases of development, indicating a strong focus on research and development.
The approved drugs for target PDE3 cover a wide range of indications, including heart failure, arterial occlusive diseases, cerebral infarction, asthma, intermittent claudication, thrombocythemia, essential, multiple sclerosis, myocardial infarction, neuralgia, cerebral hemorrhage, peripheral nervous system diseases, cerebrovascular disorders, thrombocytosis, thromboangiitis obliterans, pulmonary disease, chronic obstructive, hypertension, pulmonary, cervical myelopathy, low cardiac output, spinal cord diseases, and amyotrophic lateral sclerosis.
The rapid progression of small molecule drugs indicates intense competition around the innovative drug. However, there is limited information available regarding the research and development institutions of these biosimilars.
Countries such as China, Japan, the United States, the European Union, South Korea, Russia, France, Ukraine, Chile, Iceland, Norway, Turkey, New Zealand, Mexico, Israel, Venezuela, Argentina, Brazil, the United Kingdom, and Canada are actively developing drugs targeting PDE3. China has shown significant progress in terms of drug development for PDE3.
Overall, the current competitive landscape of target PDE3 is dynamic, with multiple companies and countries actively involved in research and development activities. The future development of target PDE3 holds promising opportunities for the pharmaceutical industry, especially in the areas of small molecule drugs and indications such as heart failure, arterial occlusive diseases, and cerebral infarction.
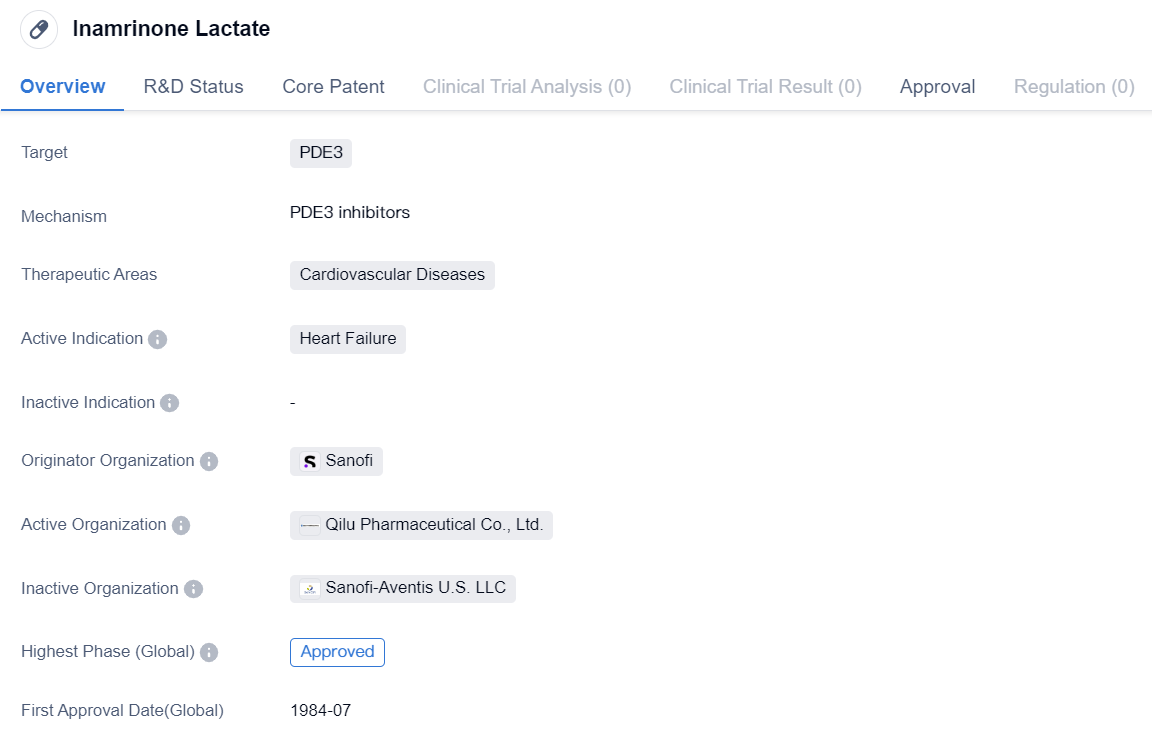
Key Drug: Inamrinone Lactate
Inamrinone Lactate is a small molecule drug that falls under the therapeutic area of cardiovascular diseases. It specifically targets PDE3, which is an enzyme involved in regulating cellular processes in the heart. The drug is primarily indicated for the treatment of heart failure, a condition characterized by the heart's inability to pump blood effectively.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The originator organization of Inamrinone Lactate is Sanofi, a multinational pharmaceutical company. The drug has achieved the highest phase of approval both globally and in China, indicating its established safety and efficacy profile. Inamrinone Lactate received its first approval in the United States in July 1984, making it a well-established medication in the field of cardiovascular medicine.
As a small molecule drug, Inamrinone Lactate is designed to interact with specific molecular targets in the body, in this case, PDE3. By inhibiting this enzyme, the drug helps to increase the levels of cAMP in cardiac cells. This, in turn, leads to enhanced contractility and relaxation of the heart muscle, improving its pumping function.
Heart failure is a chronic condition that affects millions of people worldwide. It is characterized by symptoms such as shortness of breath, fatigue, and fluid retention. Inamrinone Lactate offers a therapeutic option for patients suffering from heart failure, helping to alleviate their symptoms and improve their quality of life.
Since its approval in 1984, Inamrinone Lactate has been widely used in clinical practice, demonstrating its efficacy and safety in managing heart failure. The drug's approval in both the global and Chinese markets highlights its relevance and acceptance in different healthcare systems.
In summary, Inamrinone Lactate is a small molecule drug developed by Sanofi for the treatment of heart failure. It targets the enzyme PDE3 and has achieved the highest phase of approval globally and in China. With its first approval in the United States in 1984, Inamrinone Lactate has established itself as a valuable therapeutic option for patients suffering from cardiovascular diseases.
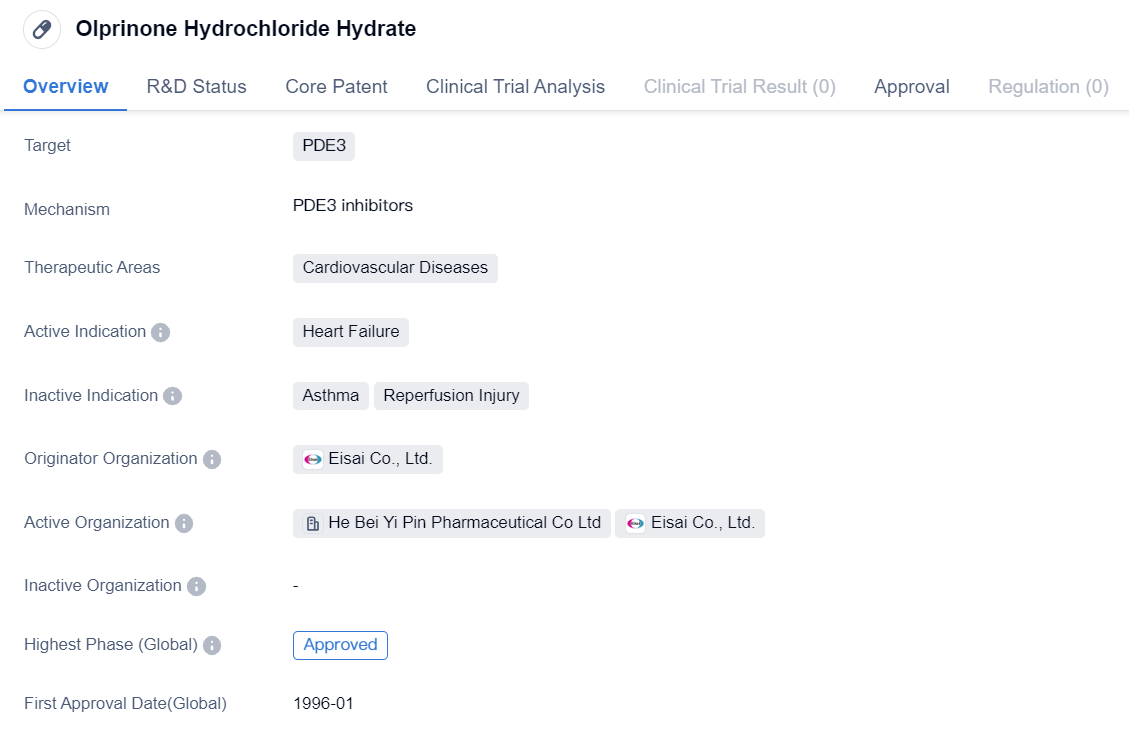
Olprinone Hydrochloride Hydrate
Olprinone Hydrochloride Hydrate is a small molecule drug that falls under the therapeutic area of cardiovascular diseases. It specifically targets PDE3. The drug is primarily indicated for the treatment of heart failure.
The originator organization of Olprinone Hydrochloride Hydrate is Eisai Co., Ltd., a pharmaceutical company based in Japan. The drug has achieved the highest phase of approval both globally and in China.
Olprinone Hydrochloride Hydrate received its first approval in January 1996 in Japan. It is important to note that the drug's first approval was specifically in Japan and not in any other country or location.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
As a small molecule drug, Olprinone Hydrochloride Hydrate is designed to interact with specific molecular targets in the body, in this case, PDE3. By targeting this enzyme, the drug aims to regulate the levels of cAMP and cGMP, which play crucial roles in cardiovascular function.
Heart failure is a condition characterized by the heart's inability to pump blood efficiently, leading to symptoms such as shortness of breath, fatigue, and fluid retention. Olprinone Hydrochloride Hydrate is indicated for the treatment of heart failure, suggesting that it may help improve cardiac function and alleviate symptoms associated with this condition.
The approval of Olprinone Hydrochloride Hydrate in both Japan and China indicates that it has undergone rigorous testing and evaluation to demonstrate its safety and efficacy. This approval status also suggests that the drug has met the necessary regulatory requirements in these countries.
Overall, Olprinone Hydrochloride Hydrate is a small molecule drug developed by Eisai Co., Ltd. for the treatment of heart failure. Its approval in Japan and China highlights its potential as a therapeutic option for patients with this condition. Further research and clinical studies may be conducted to explore its effectiveness in other regions and indications within the cardiovascular disease field.