Carisma Therapeutics Offers Corporate Progress Report and Presents Q4 and Annual Financials for 2023
Carisma Therapeutics Inc., a biopharmaceutical enterprise in the clinical phase dedicated to the innovation and progression of novel immunotherapy treatments, has disclosed its financial performance for the last quarter as well as the cumulative annual figures up to December 31, 2023. Additionally, the company offered an update on its corporate activities.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
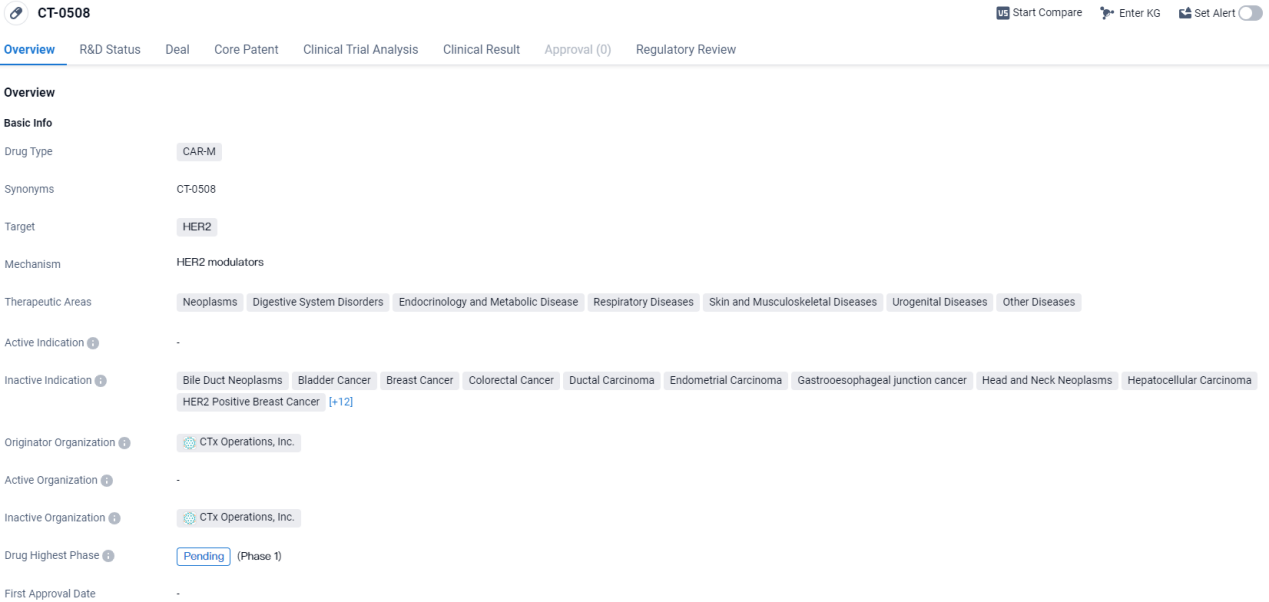
Results obtained from the evaluation of CT-0508 within Study 101 indicate that CAR-M therapy is acceptable in terms of safety, can be produced effectively, and exhibits activity in cell-regulating processes within HER2 positive solid tumors. Steven Kelly, Carisma's CEO and President, remarked, "The fusion of these findings with the numerous inherent benefits of a monocyte-driven strategy observed during early-stage studies strengthens our belief in the groundbreaking potential of CT-0525 within the realm of CAR-M interventions."
Kelly further noted, "Following a strategic assessment of our company's direction, we have decided to concentrate on pipeline projects that promise the most compelling results and are nearing key progress milestones. As a result, we will be taking measures aimed at optimizing resources, which include reorganizing our team structure. My heartfelt thanks go out to the employees affected by these changes for their dedication to our core goals and their unwavering commitment to patient care."
In September 2023, Carisma disclosed early-stage results regarding safety, endurance, as well as production capability for CT-0508, gathered from an initial group of 14 participants enrolled in the open-label, Phase 1 clinical assessment. Additionally, this study aimed to determine several prespecified secondary outcomes related to the clinical investigation.
Furthermore, a total of six patients have been incorporated into a collateral inquiry of Study 101 that investigates the simultaneous administration of CT-0508 with pembrolizumab, an inhibitor of the programmed cell death protein 1. This examination aims to ensure the safety and endurance of the combined use, together with an evaluation of various prespecified secondary outcomes.
While the company is committed to maintaining the ongoing Study 101 for current enrollees, it intends to halt the progression of new participant entry into both the primary study and its related secondary examinations. Carisma anticipates sharing results from the substudy evaluating the combined use of CT-0508 and pembrolizumab in the second quarter of 2024.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
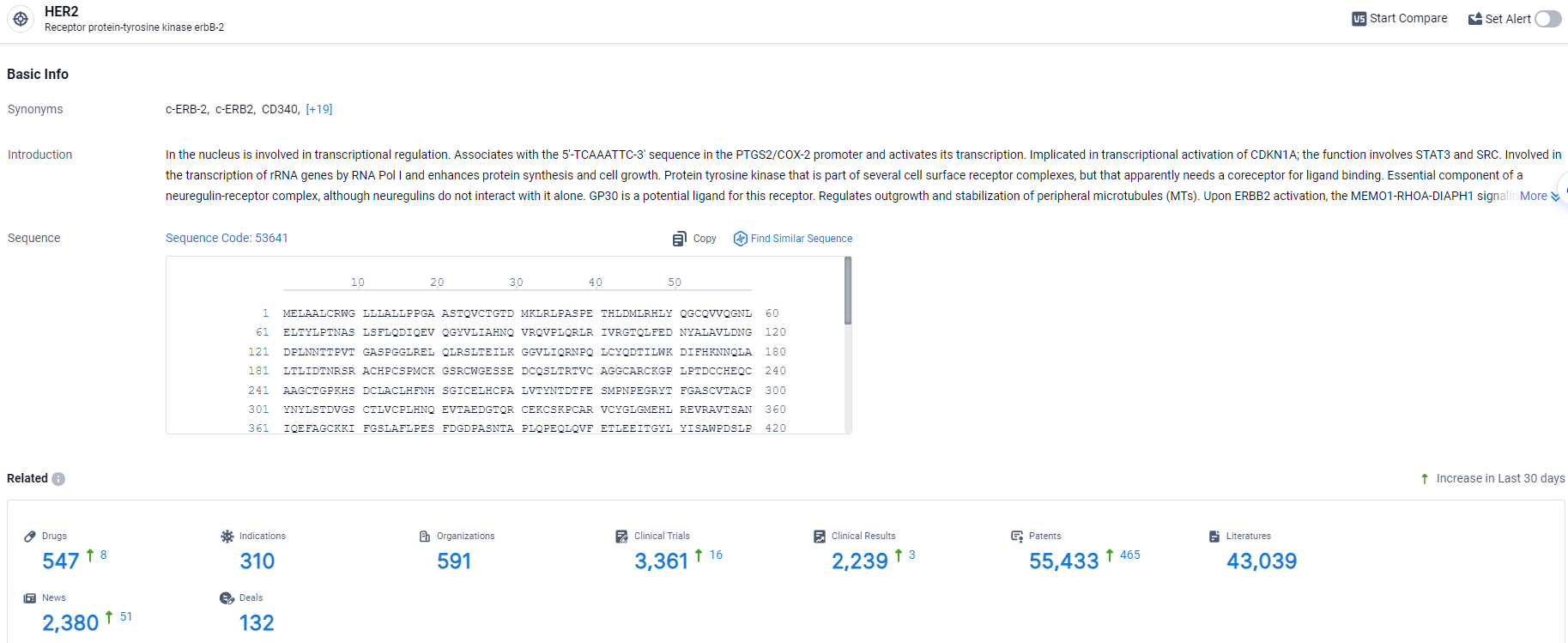
According to the data provided by the Synapse Database, As of April 3, 2024, there are 547 investigational drugs for the HER2 target, including 310 indications, 591 R&D institutions involved, with related clinical trials reaching 3361, and as many as 55433 patents.
CT-0508 targets HER2 and is being investigated for its potential applications in various disease areas. The drug is currently in the pending phase of development and has been designated as a Fast Track drug, indicating its potential to address unmet medical needs.