Coherus announces FDA approval for UDENYCA ONBODY™, a cutting-edge pegfilgrastim-cbqv delivery technology
Coherus BioSciences, Inc., which operates as a commercial biopharmaceutical enterprise emphasizing the innovation, production, and marketing of new immunotherapy treatments for cancer, has reported that the U.S. Food and Drug Administration has given the nod to its UDENYCA ONBODY™ device. This device is an on-body injector format of the company’s product, UDENYCA® (pegfilgrastim-cbqv), a biosimilar to pegfilgrastim. It is intended for use subsequent to chemotherapy sessions to reduce the likelihood of infections indicated by the occurrence of febrile neutropenia.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"The newly launched UDENYCA ONBODY injector is the culmination of extensive investment in R&D to create a distinctive, cutting-edge device that offers patients an automated way to administer their prescriptions," stated Denny Lanfear, CEO at Coherus. "Patients dealing with cancer, alongside their healthcare providers, can now select the most suitable form of UDENYCA delivery, whether it be a preloaded syringe, our auto-injector, or the novel on-body injector."
Paul Reider, the Chief Commercial Officer at Coherus, explained, "Our market analysis identified a substantial market need for an innovative on-body pegfilgrastim administration system that caters to the varied requirements of patients. We anticipate that the UDENYCA ONBODY's quick five-minute dispensing period and its state-of-the-art, retracting needle feature will receive positive feedback from those undergoing cancer treatment, their caretakers, and healthcare professionals."
Rich Hameister, the Chief Technical Officer at Coherus, highlighted, "Our advanced UDENYCA ONBODY injector stands out as it was specially engineered for its intended medical application, rather than being a modified version of an existing product. It represents a fresh, holistic, and original design strategy for pegfilgrastim delivery that integrates exclusive technology and user-centric design insights to ensure dependability and ease of use for the patient."
The UDENYCA ONBODY has been thoughtfully crafted with the end-user's comfort and safety as a priority. It is equipped with features such as visual indicators, acoustic confirmation signals that reassure the user of successful administration, and a robust adhesive that is generally well-accepted by the skin. Upon completion of the injection, the needle retracts on its own, minimizing the chance of accidental needlesticks.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
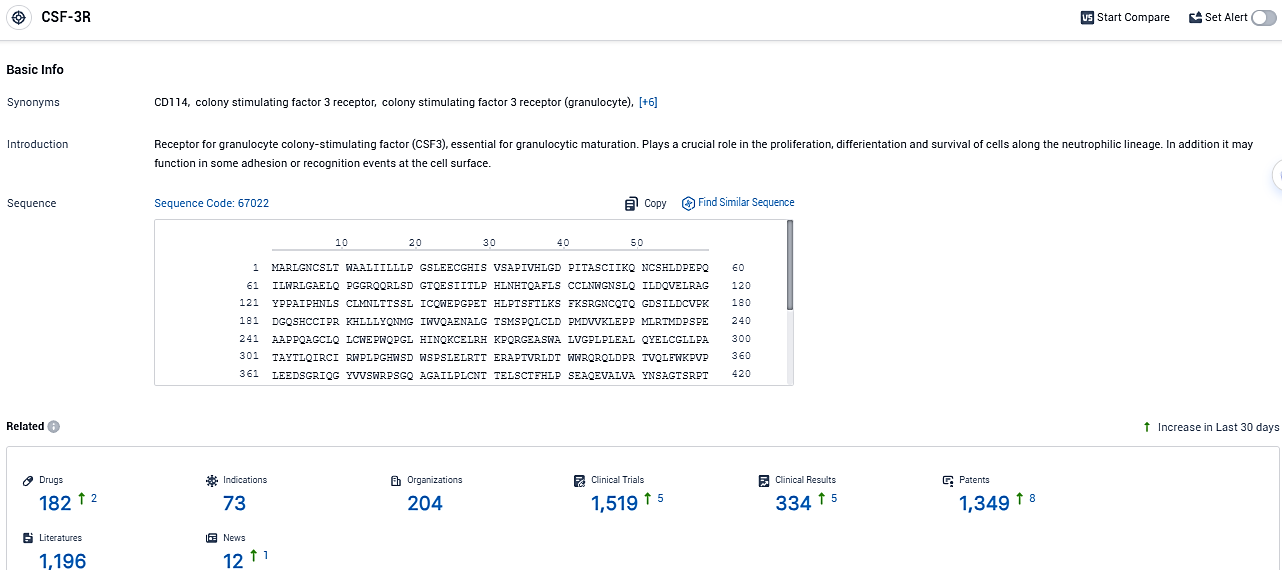
According to the data provided by the Synapse Database, As of December 30, 2023, there are 182 investigational drugs for the CSF-3R target, including 73 indications, 204 R&D institutions involved, with related clinical trials reaching 1519, and as many as 1349 patents.
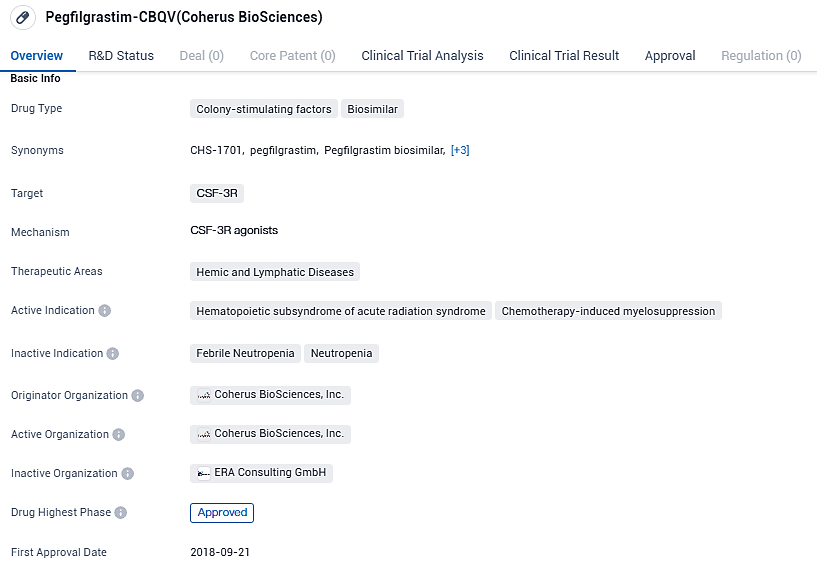
Pegfilgrastim-CBQV is a biosimilar drug developed by Coherus BioSciences, Inc. It has been approved for the treatment of hematopoietic subsyndrome of acute radiation syndrome and chemotherapy-induced myelosuppression. The drug targets the CSF-3Rreceptor and works by stimulating the production of white blood cells in the bone marrow. Its first approval occurred in September 2018 in multiple European countries, indicating its safety and efficacy in these regions.