CSF-1R inhibitor——the new layout of macrophage-targeted anticancer drugs
Tumor-associated macrophages (TAMs) can inhibit tumor immune efficacy. Colony stimulating factor 1 receptor (CSF-1R)/CSF-1 pathway involved in regulating the function of TAMs. CSF-1R is a tyrosine kinase transmembrane receptor, also a member of the growth factor CSF-1/platelet derived growth factor (PDGF) receptor family.
This receptor family has inherent tyrosine-specific protein kinase activity. CSF-1R participates in the survival, proliferation, differentiation, recruitment, and function of mononuclear phagocytes (such as macrophages, monocytes). IL-34 has biological characteristics similar to CSF-1, but their main differences come from different spatio-temporal regulation. In addition, CSF-1 can function through autocrine and paracrine methods, while IL-34 only works locally.
High levels of CSF-1 have been reported in breast cancer, pancreatic cancer, prostate cancer, ovarian cancer, kidney cancer, and many other types of cancers. Overexpression of CSF-1 and its receptor CSF-1R in tumors is associated with poor prognosis. Therefore, small molecule inhibitors and antibodies against CSF-1R are currently being developed clinically, which show anti-tumor activity.
Competitive landscape
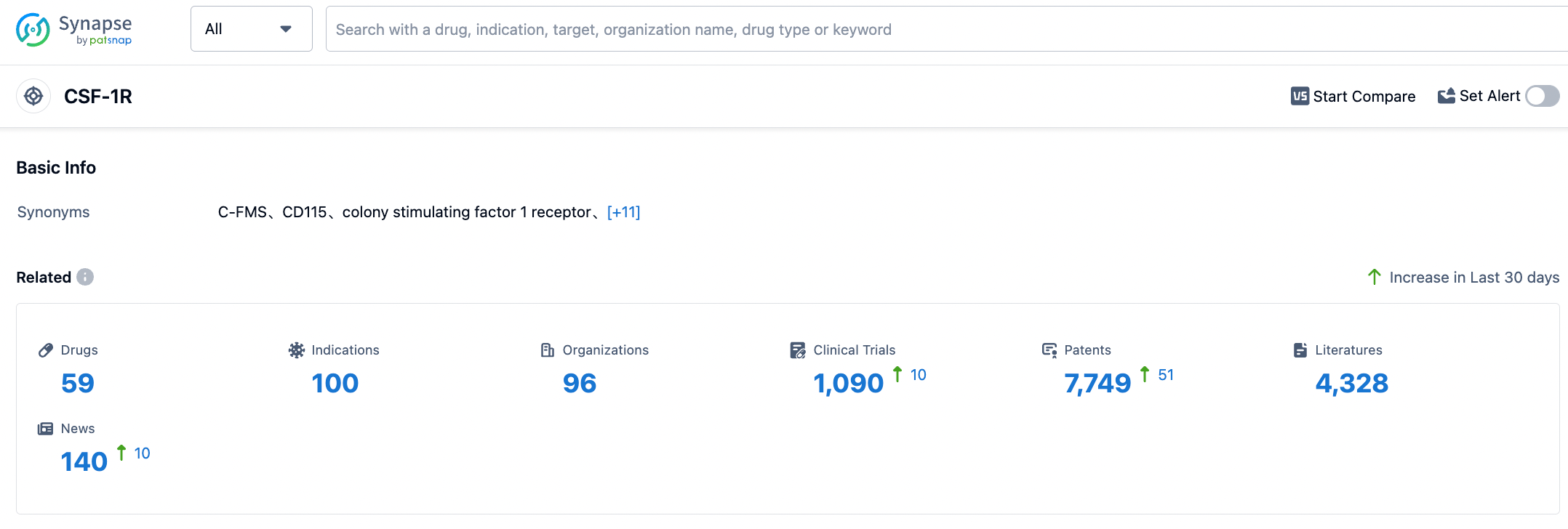
According to the data from Synapse, there are currently 59 drugs targeting CSF-1R under development, covering 100 different disease areas, with related research institutions reaching 96, and there are 1090 clinical trial results related to CSF-1R-targeted drugs. In addition, the development of CSF-1R-targeted drugs has been supported by 7749 patents, indicating innovation and technological breakthroughs in this field. The research and development of CSF-1R-targeted drugs have also received wide attention and protection, and 4328 related research papers have been published. These papers summarize the important findings in the research and development progress, mechanism research, and clinical application of CSF-1R-targeted drugs.
Key Drug
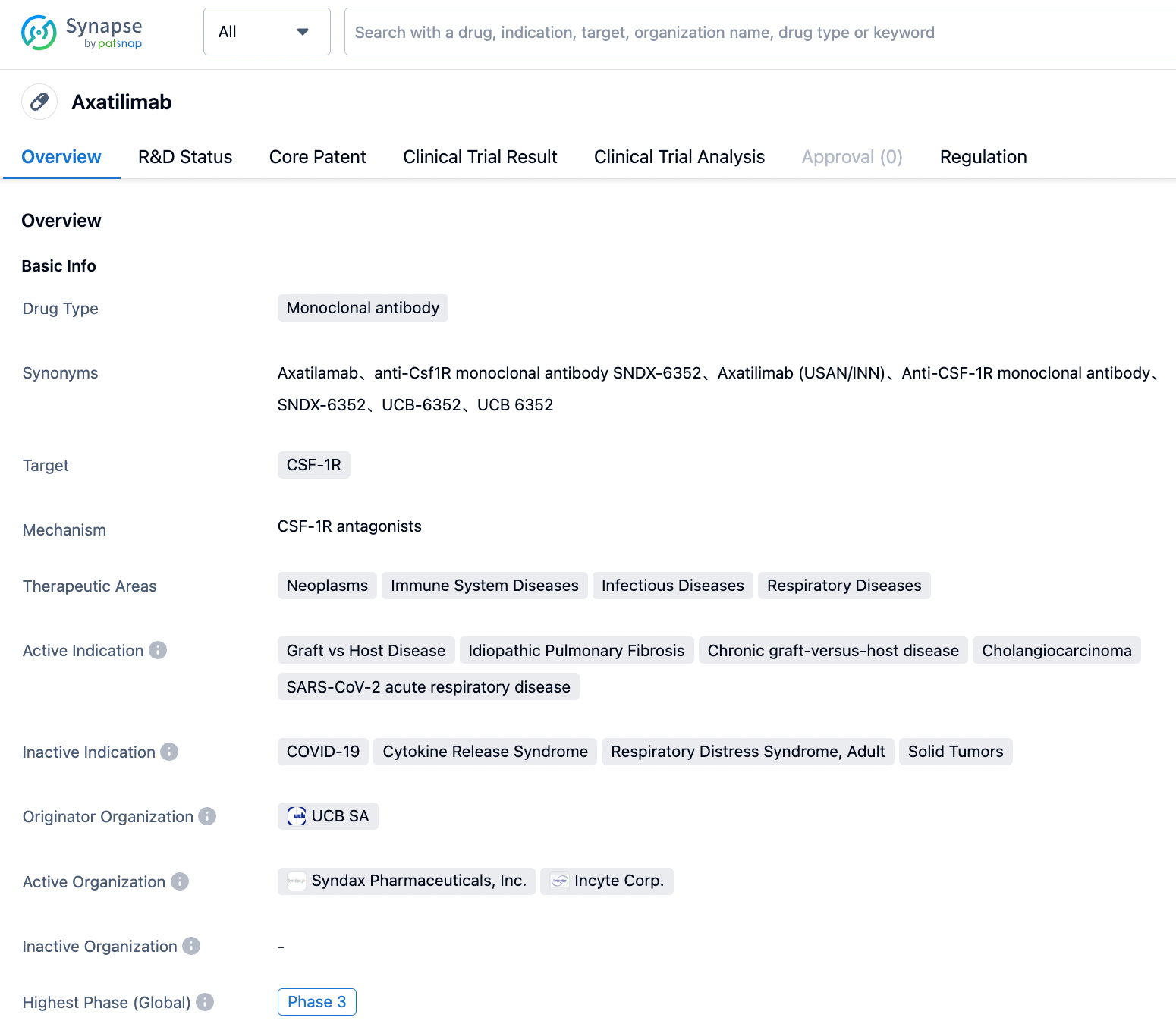
Axatilimab
Axatilimab (SNDX-6352) is a selective CSF-1R inhibitor developed by Incyte and Syndax Pharmaceuticals, mainly used for the treatment of chronic graft versus host disease (cGVHD)(Phase 3), Idiopathic Pulmonary Fibrosis (Phase 3), and cholangiocarcinoma (Phase 2) among other diseases. CSF-1 has been proven to stimulate the growth and differentiation of donor-derived monocytes into macrophages, thereby stimulating the activation of fibroblasts and the production of collagen proteins, leading to the manifestations observed in various organs of cGVHD patients. The use of axatilimab to block CSF-1R may reduce the number of these inflammatory macrophages and play a crucial role in the treatment of chronic graft versus host disease.
According to results from a phase 1/2 clinical study AGAVE-201, an overall response rate (ORR) of 68% was observed in two dosage groups utilized for further research. A clinically meaningful improvement in symptoms was reported in 53% of patients, as assessed by the Lee Symptom Scale, and 43% of patients continued to receive treatment at the time of data cut-off. The most recent results demonstrated that, out of 40 enrolled patients, the regulatory authority approved endpoint ORR reached 82%, and 58% of patients reported a significant improvement in cGVHD-related symptoms using the Lee Symptom Scale.
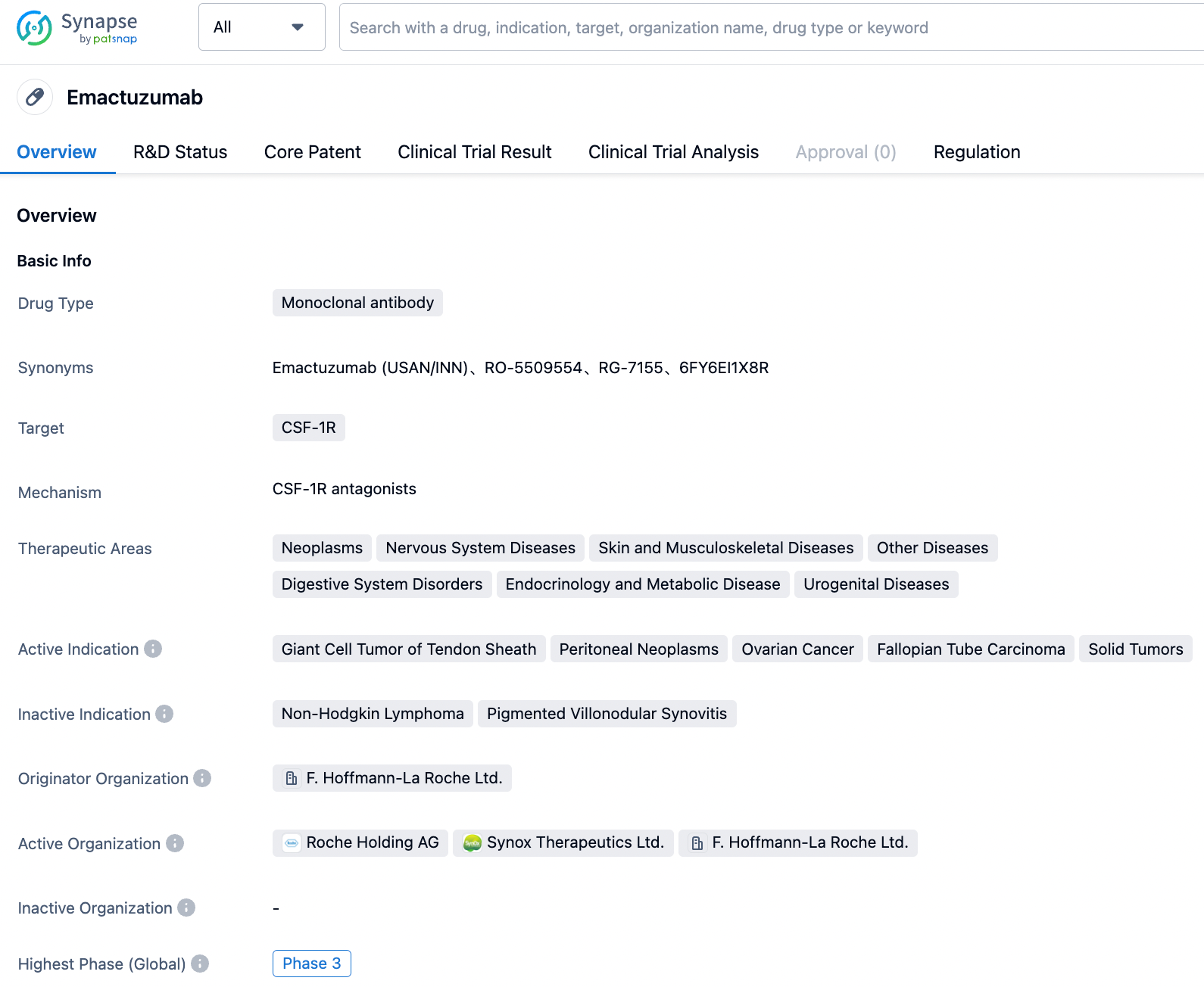
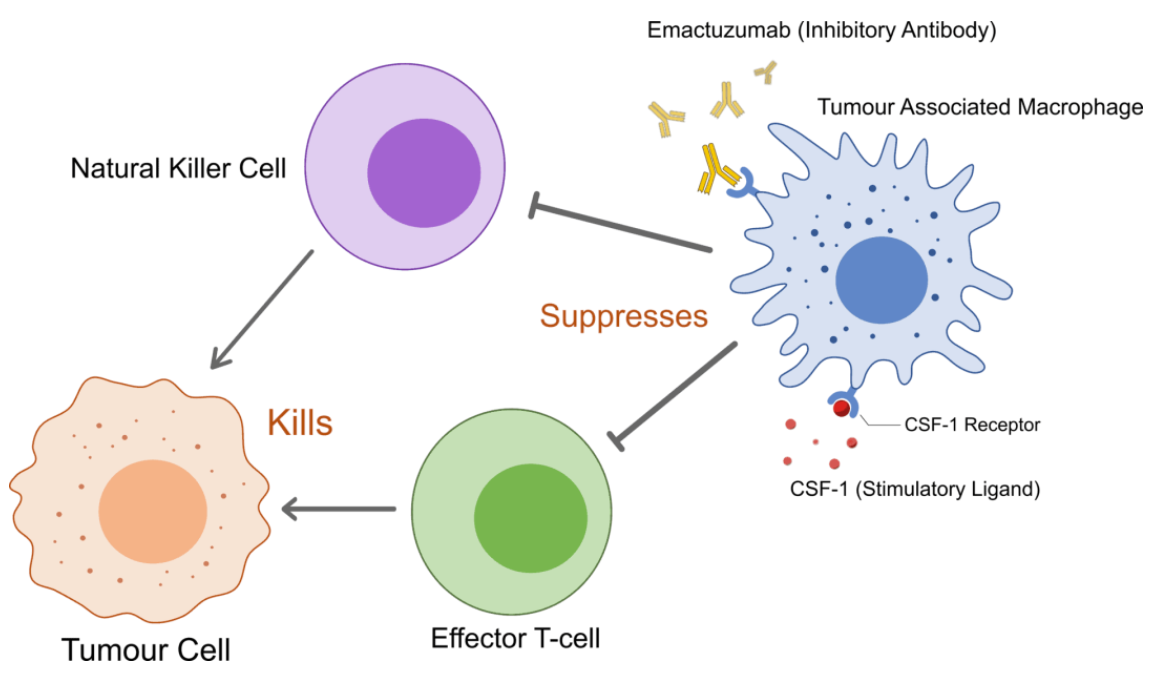
Emactuzumab
Emactuzumab (RG-7155) is a CSF-1R inhibitor developed collaboratively by Roche and Celleron Therapeutics and is primarily used to treat tenosynovial giant cell tumor (Phase 3), ovarian cancer (phase 2), and peritoneal malignant tumors (phase 2) among others. As a leading therapeutic antibody against CSF-1R, emactuzumab functions by targeting and eliminating tumor-associated macrophages (TAMs). The drug has shown high safety in patients and significant efficacy on diffuse Tenosynovial giant cell tumor (TGCT), a rare disease characterized by the abnormal proliferation of macrophages in the synovial tissue of joints and tendons.
According to a clinical study conducted on patients with diffuse TGCT, the overall response rate of Emactuzumab was 71% (45/63), and the disease control rate was 98% (62/63). In 1-year and 2-year follow-up, MRI scans showed that 19/27 (70%) and 9/14 (64%) of patients respectively continued to respond to the treatment, indicating that Emactuzumab has a long-lasting effectiveness.
In contrast to the early approved CSF-1R multi-target inhibitors like Surufatinib, Pexidartinib, Vorolanib, etc., the new generation CSF-1R inhibitors have a better selectivity. They can significantly reduce the side effects of off-target effects and show good efficacy in clinical trials. The development of small molecule drugs has further enhanced patient's oral convenience, such as Pimicotinib from Handi Pharmaceuticals. In addition to playing a crucial role in the field of anti-cancer treatments, CSF-1R inhibitors also have wide application prospects in many areas including inflammation-related diseases and skeletal-related diseases. In these indications, the application prospect of CSF-1R inhibitors will be further expanded and explored. Through continuous exploration and innovation, CSF-1R inhibitors are expected to become an important therapeutic choice in multiple fields, bringing more hope and well-being to patients.