Cytokinetics Reports Positive Phase 3 SEQUOIA-HCM Trial Results for Aficamten in Obstructive Hypertrophic Cardiomyopathy
Cytokinetics, Incorporated has declared encouraging preliminary outcomes from the critical Phase 3 study, SEQUOIA-HCM. This clinical trial, whose full name stands for Safety, Efficacy, and Quantitative Understanding of Obstruction Impact of aficamten in HCM, mainly focused on evaluating aficamten in individuals experiencing symptomatic obstructive hypertrophic cardiomyopathy.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Findings from the SEQUOIA-HCM study indicate that aficamten has a considerable positive impact on the ability to perform physical activities when compared to a placebo, demonstrated by an augmentation in the maximal volume of oxygen consumption ascertained through cardiopulmonary exercise testing, with a least square mean enhancement of 1.74 mL/kg/min, which is statistically significant (p=0.000002).
Administering aficamten showed a homogeneous benefit throughout every pre-established subgroup analysis, which encompassed a variety of baseline characteristics specific to patients and their corresponding therapeutic approaches, whether they incorporated beta-blocker therapy or not in their regime.
Dr. Fady I. Malik, Executive Vice President of Research & Development at Cytokinetics shared his enthusiasm about the outcome, confirming that the data lived up to their expectations in terms of both potency and safety profile. According to him, adding aficamten to existing treatment protocols noticeably influenced patients' exercise capabilities, alongside providing quick and prolonged improvement in both symptoms and patients' functional class dealing with obstructive HCM, all while preserving the anticipated safety and tolerability profile.
Dr. Malik expressed gratitude towards everyone involved in SEQUOIA-HCM, including patients, researchers, and clinical site staff for their contribution to the study and the company's broader clinical research endeavors. He also expressed eagerness about presenting comprehensive findings from this study at an upcoming medical conference slated for 2024.
Dr. Martin Maron, a leading authority on Hypertrophic Cardiomyopathy from Lahey Hospital and Medical Center, and Tufts University School of Medicine, and also the National Principal Investigator for SEQUOIA-HCM, expressed his optimism about the potential of cardiac myosin inhibitors as novel treatments for patients with symptomatic obstructive HCM, (HCM). He commended the striking outcomes observed in the SEQUOIA-HCM trial.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
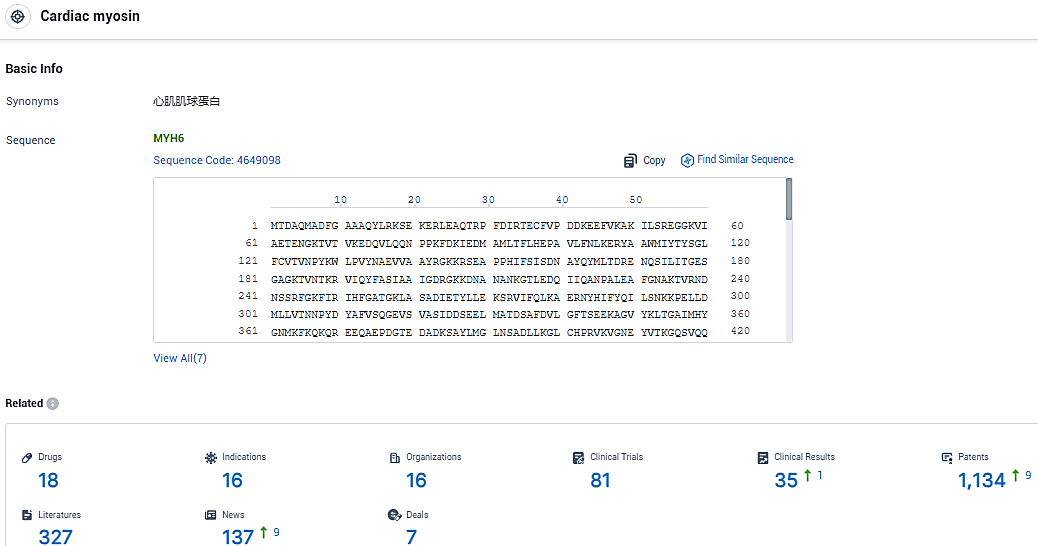
According to the data provided by the Synapse Database, As of January 04, 2024, there are 18 investigational drugs for the cardiac myosin target, including 16 indications, 16 R&D institutions involved, with related clinical trials reaching 81, and as many as 1134 patents.
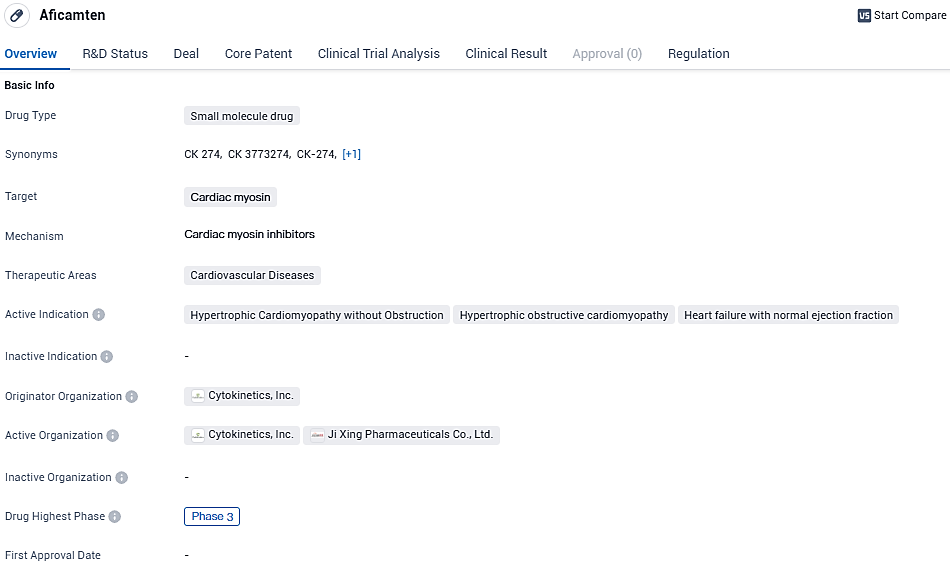
Aficamten's development as a small molecule drug targeting cardiac myosin for the treatment of cardiovascular diseases, including hypertrophic cardiomyopathy without obstruction, hypertrophic obstructive cardiomyopathy, and heart failure with normal ejection fraction. The drug is currently in Phase 3 of clinical development globally and in China. Additionally, Aficamten has received orphan drug and breakthrough therapy designations, highlighting its potential as an innovative and promising treatment option in the field of biomedicine.