Decoding MCLA-129: a comprehensive study of its R&D trends and its clinical results in 2023 ESMO_ASIA
On 3 Dec 2023, the latest clinical result of MCLA-129, an anti-EGFR/c-MET bispecific antibody, for the treatment of head and neck squamous cell cancer (HNSCC) was reported in 2023 ESMO_ASIA.
MCLA-129's R&D Progress
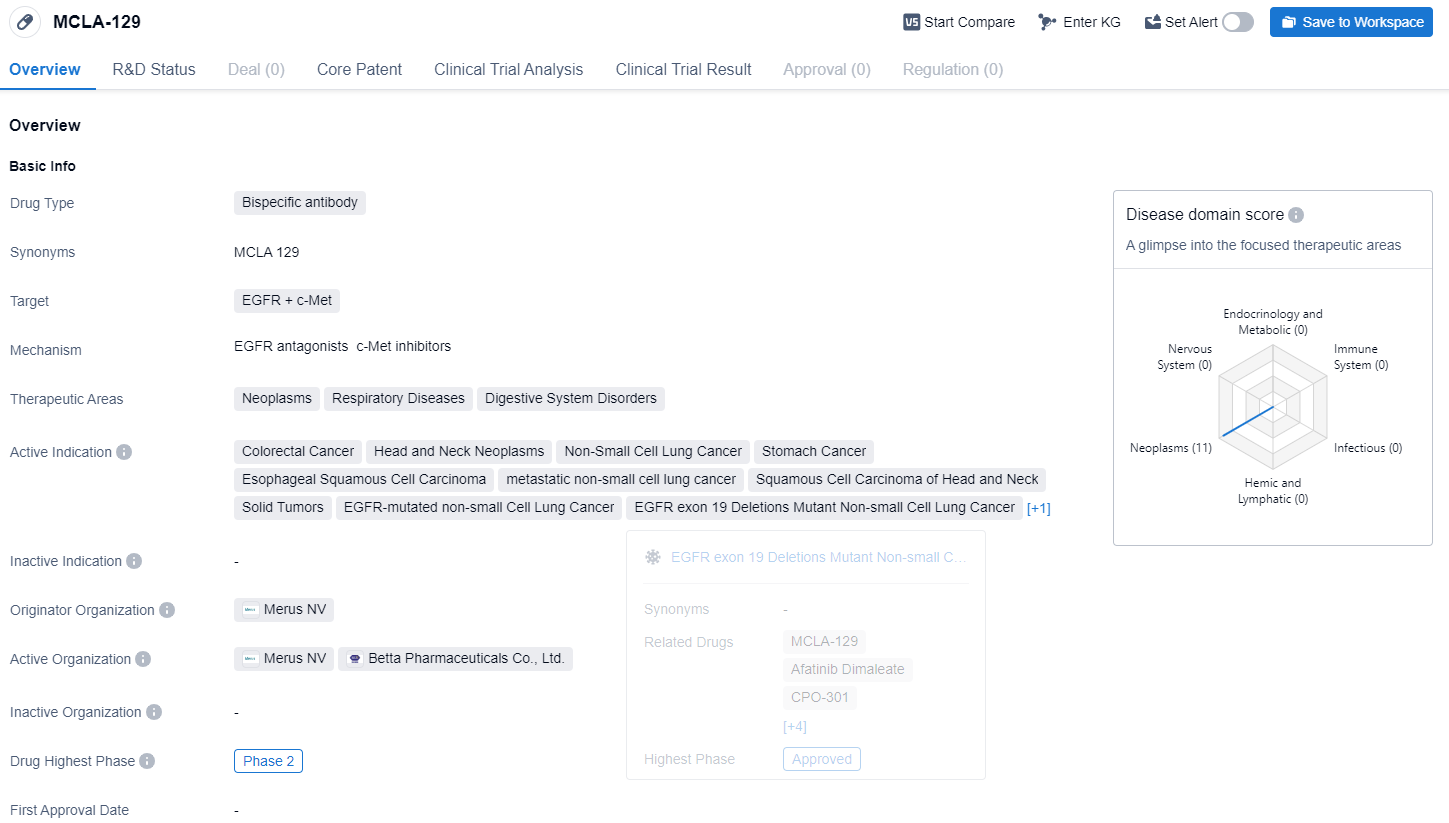
MCLA-129 is a bispecific antibody drug that targets both EGFR (epidermal growth factor receptor) and c-Met. MCLA-129 targets a variety of health issues, including cell growth abnormalities, lung-related ailments, and digestive tract problems. Its research focus encompasses an array of cancers, notably colorectal, head and neck, and several types of non-small cell lung cancer (NSCLC), such as those with EGFR mutations, EGFR exon 19 deletions, and EGFR exon 21 substitutions. Additionally, it is explored for efficacy against stomach and esophageal cancers, metastatic NSCLC, and other solid tumors.
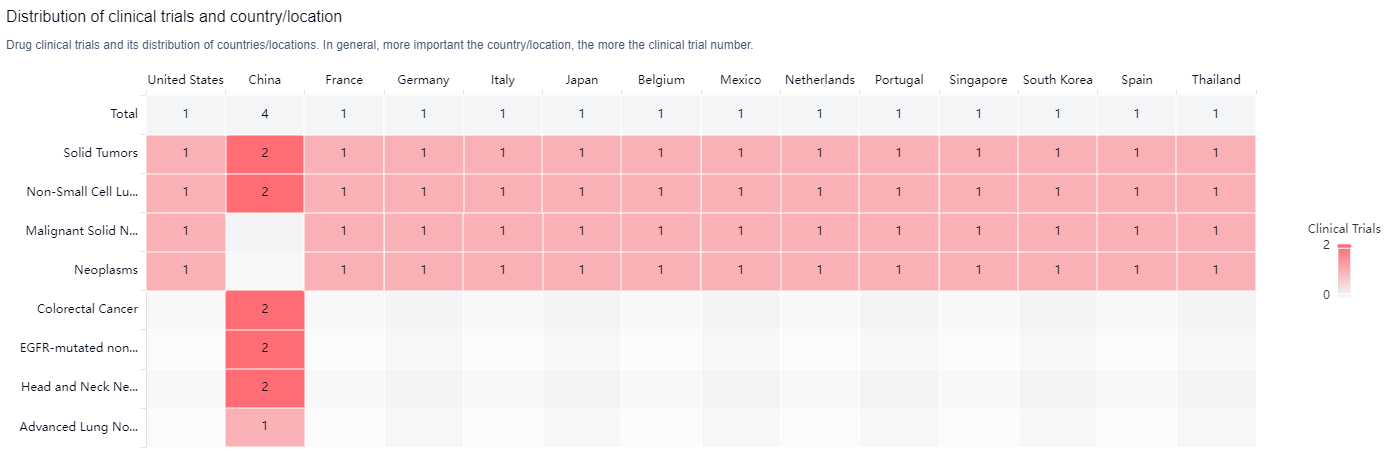
According to the Patsnap Synapse, MCLA-129 is currently in Phase 2 of clinical trials globally. And the clinical trial distributions for MCLA-129 are primarily in the United States, China and France. The key indication is Solid Tumors.
Detailed Clinical Result of MCLA-129
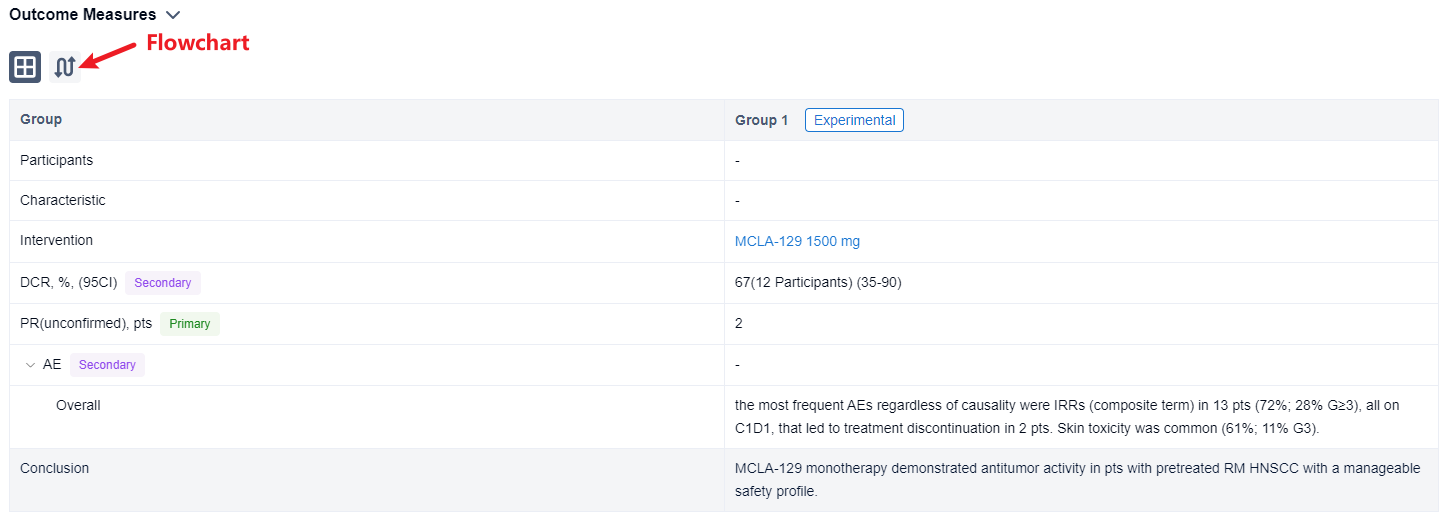
This non-randomized, parallel assignment, open-labeled clinical trial (NCT04868877,EUCTR2021-000203-20-BE) was aimed to investigate the efficacy and safety of MCLA-129 in head and neck squamous cell cancer (HNSCC).
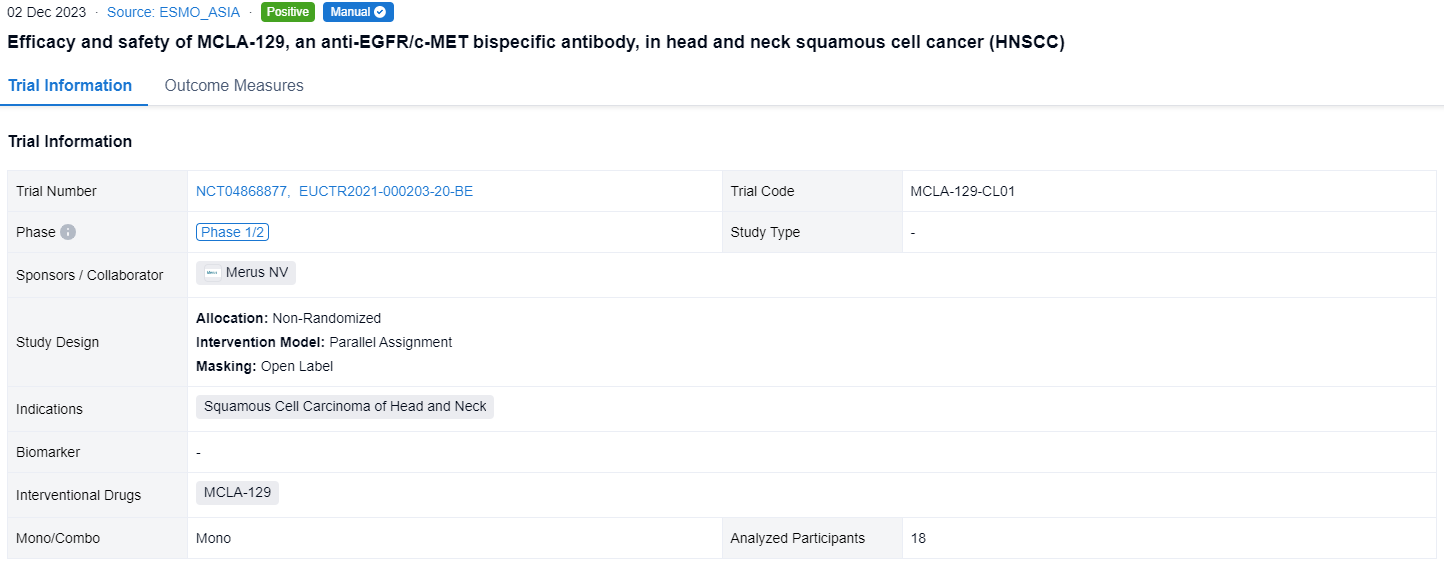
In this study, pts with RM HNSCC who relapsed on or were not candidates for approved therapies, were enrolled. Pts received MCLA-129 1500 mg IV Q2W, until disease progression or unacceptable toxicity. Tumor imaging was conducted Q8W. The primary endpoint is investigator-assessed ORR, per RECIST 1.1, evaluated in pts with measurable disease at baseline, ≥2 MCLA-129 cycles and ≥1 post-baseline scan. Secondary endpoints include DCR and safety. Biomarker analyses of baseline EGFR/c-MET expression and ctDNA mutation status are planned.
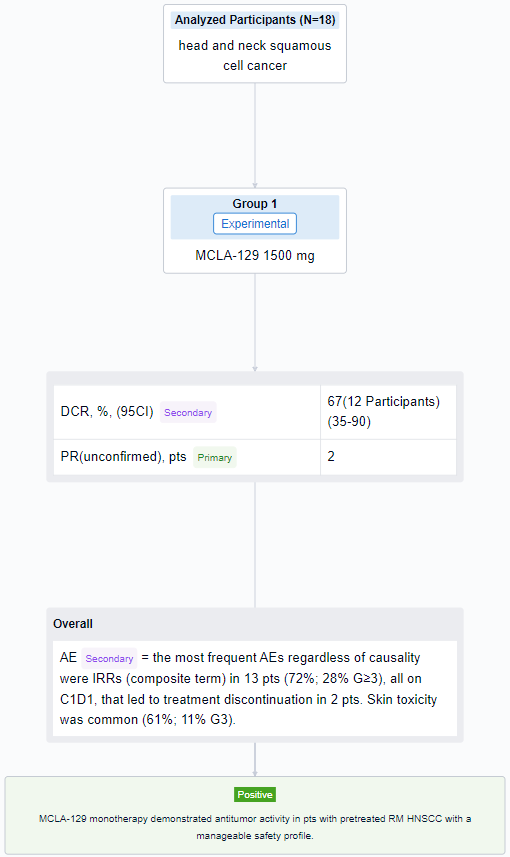
The result showed that as of 10 May 2023, 18 RM HNSCC pts were treated. Median age was 62.5 y (range 32-73), 83% were male, 44%/56% of pts had ECOG PS 0/1. Primary tumor locations were nasal cavity/paranasal sinus (4 pts), oropharynx (4 pts), larynx (3 pts), other (7 pts). Pts received a median of 2 lines of prior systemic therapy, including anti-PD-(L)1 (78%) and platinum-based chemotherapy (89%); 28% received cetuximab in the RM setting. Median exposure duration was 8 wks (range 2-17); 9 pts (50%) were continuing treatment at data cutoff. Of 12 evaluable pts, 2 (17%) had unconfirmed partial responses, 1 of which was ongoing at data cutoff. The DCR was 67% (95% CI 35-90%). Among 18 pts treated, the most frequent AEs regardless of causality were IRRs (composite term) in 13 pts (72%; 28% G≥3), all on C1D1, that led to treatment discontinuation in 2 pts. Skin toxicity was common (61%; 11% G3). No cases of interstitial lung disease were reported in this cohort.
It can be concluded that MCLA-129 monotherapy demonstrated antitumor activity in pts with pretreated RM HNSCC with a manageable safety profile.
How to Easily View the Clinical Results Using Synapse Database?
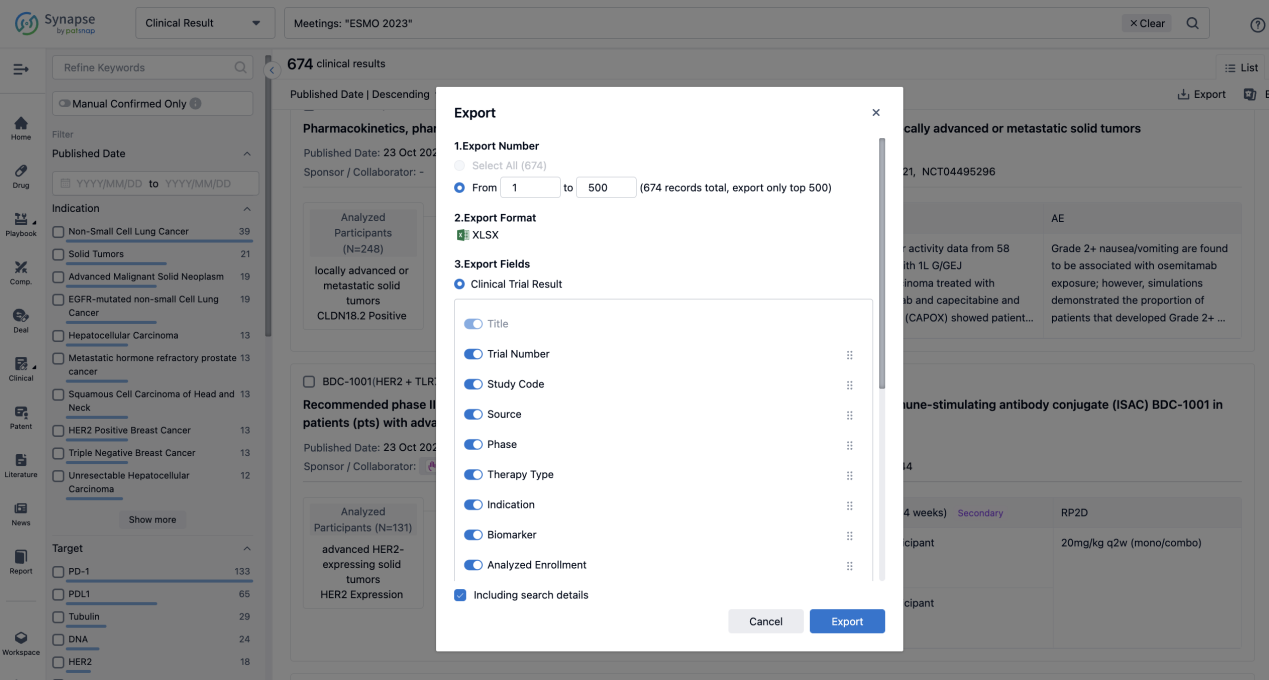
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
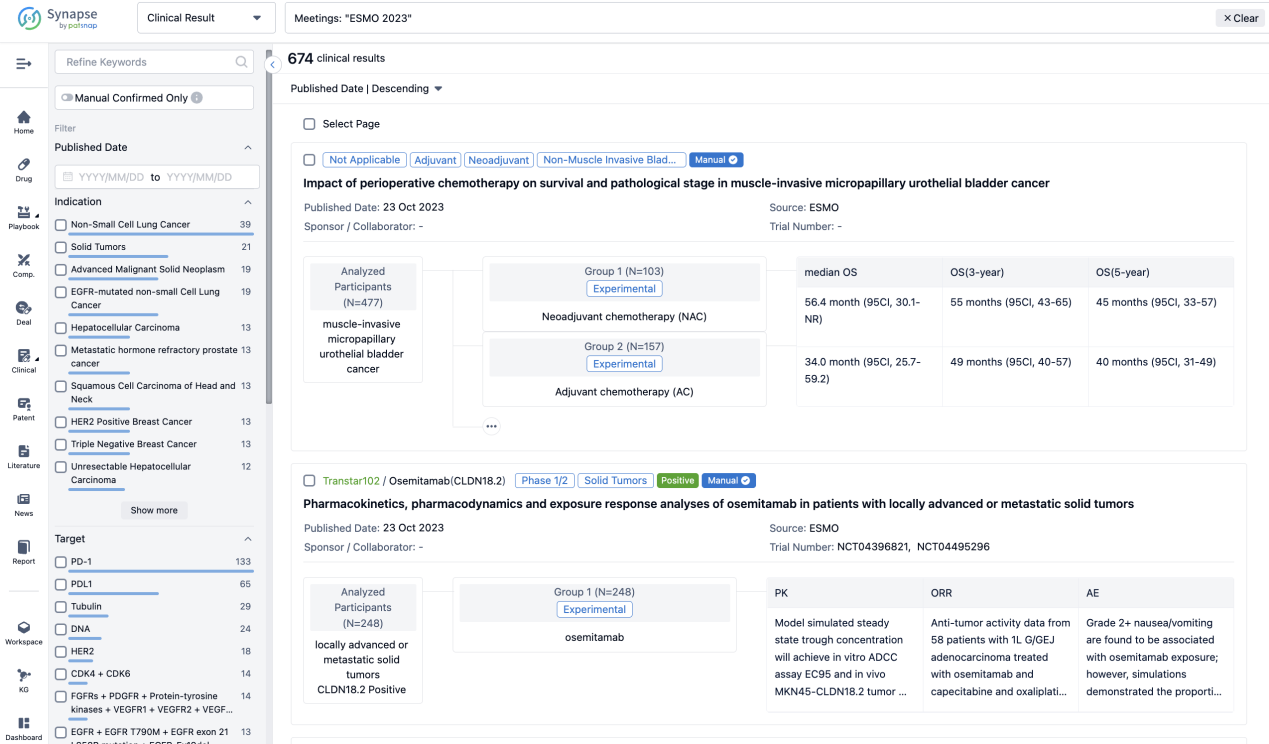
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
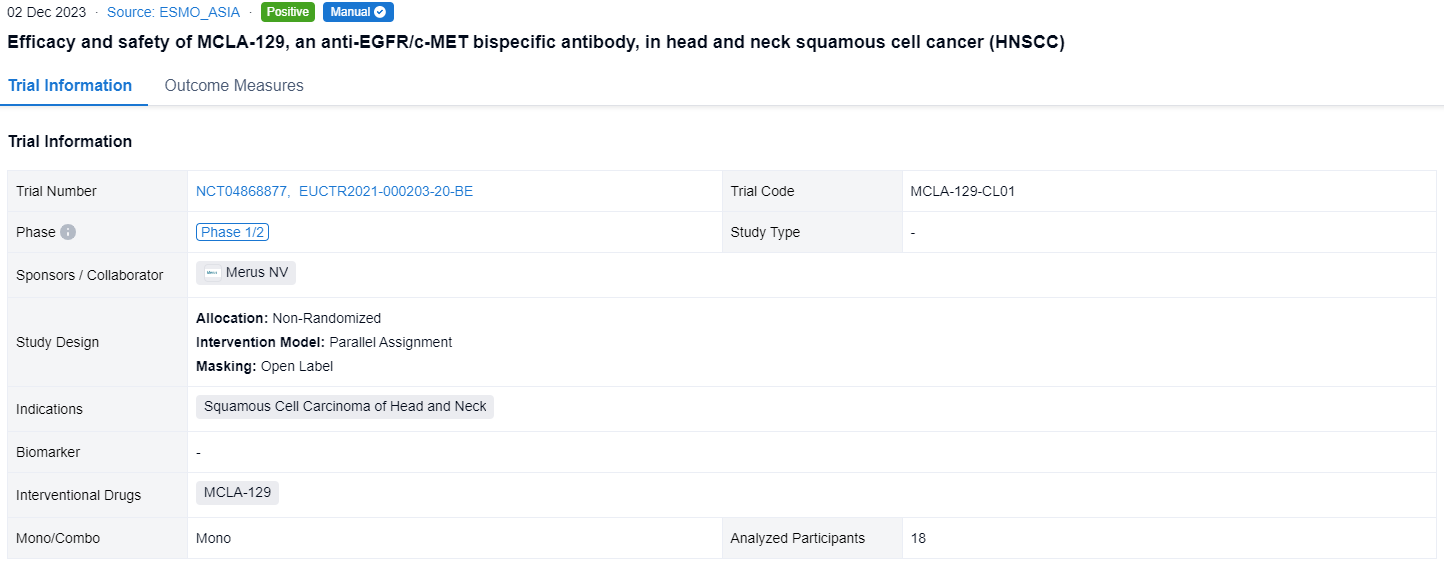
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
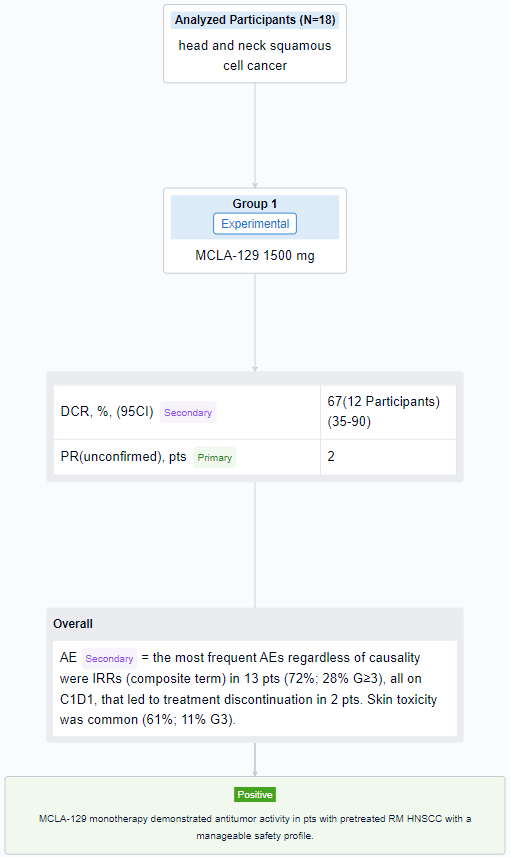
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!