Discussing AstraZeneca's Brain-penetrating KRAS Inhibitor
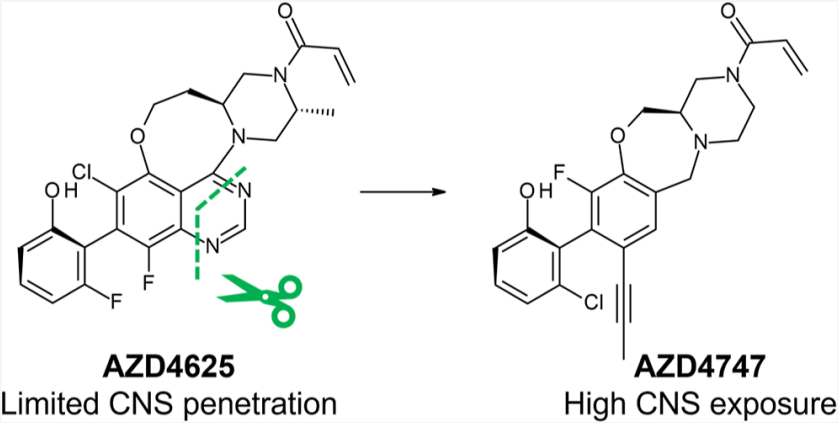
Recently, the top journal JMC updated a discovery process of the brain-penetrating KRAS G12C inhibitor AZD4747 developed by AstraZeneca. As shown in Figure 1, based on AstraZeneca's self-developed G12C inhibitor AZD4625, the planarity of the molecule was reduced by removing pyrimidine and introducing acetylmethane, yielding AZD4747 with high CNS exposure (preclinical stage).
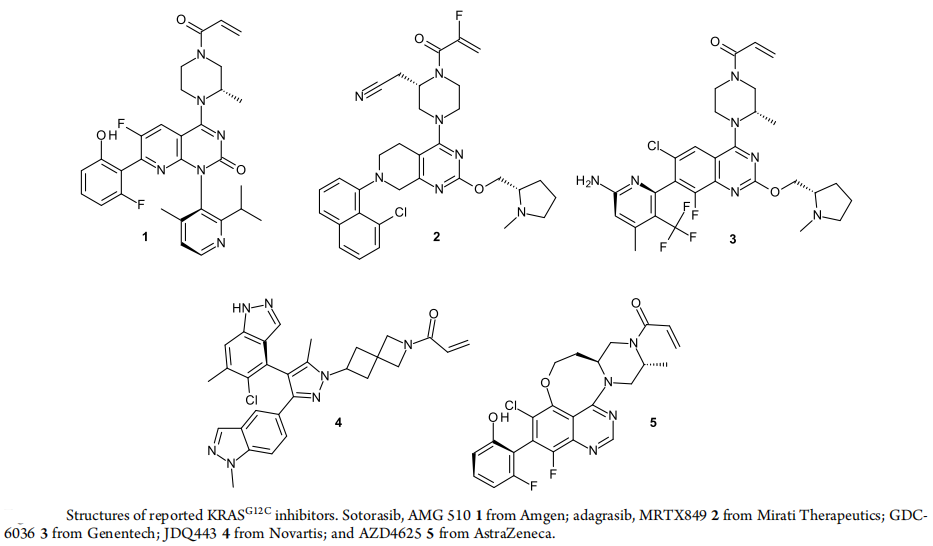
The race for KRAS G12C inhibitors is very crowded, where a variety of drugs are currently being evaluated. AMG510 and MRTX849 have already hit the market, with numerous inhibitorsin the clinical trial stage. In addition to GDC-6036 by Genetech, JDQ443 by Novartis, and AZD4625, all structure disclosed in Figure 2, there are also clinical trial drugs with undisclosed structures including LY3537982 by Eli Lilly, BI 1823911 by Boehringer Ingelheim, and D-1553 by InventisBio. Early molecules from Eli Lilly LY3499446 and Johnson & Johnson JNJ-74699157 had their clinical trials terminated due to toxicity. Other subtypes of KRAS such as G12D, G12V, G13D, etc., are also being fervently developed, with panKRAS and RAS on inhibitors also becoming a hot topic.
On July 4th, 2023, Jacobio Pharmaceuticals announced that AbbVie has issued a termination notice based on portfolio and strategic decisions, returning the global rights to the SHP2 inhibitor back to them. Novartis' SHP2 inhibitor as a single-agent clinical trial had long failed, and Jacobio Pharmaceuticals having their rights returned was only a matter of time. The combination of KRAS inhibitors and SHP2 inhibitors seem to have become the last straw for SHP2.
According to Amgen's financial report, the sales of sotorasib in 2021 and 2022 were $90 million and $285 million respectively, which were lower than the forecasts of Wall Street analysts. In clinical trials, the response rates of Sotorasib and Adagasib are around 40%, with the median progression free survival (PFS) improving by about 6 months. These results are not ideal for targeted mutant-specific treatments and are not as good as those observed for other targeted drugs (such as EGFR and ALK inhibitors).
Additionally, resistance to the treatment has appeared, with various mechanisms including mutations in genes like KRAS, NRAS, BRAF, EGFR, FGFR2, MYC, etc. Gene amplifications and point mutations have been observed in downstream effectors like KRAS, NRAS and MEK, leading to direct resistance to inhibition or reactivation through bypass signaling pathways. The presence of central nervous system (CNS) metastatic disease, especially for drugs that cannot penetrate the brain, is another key mechanism of inherent and potential acquired resistance. For example, metastatic brain disease is a common feature of NSCLC, occurring in up to 20% of patients. Control of intracranial disease can be achieved through radiotherapy, however, it is highly toxic. CNS metastasis is also common in KRASG12C-positive NSCLC, with up to 28% of patients showing central disease at diagnosis, with the rate increasing to 40% upon follow-up. Therefore, KRAS G12C inhibitors that can penetrate the brain have a differentiated clinical advantage.
Achieving drug exposure in the CNS has been a challenge for several decades and is much harder than simply achieving oral exposure. This is because the blood-brain barrier (BBB) is a powerful barrier to exogenous drugs, expressing high levels of efflux transporters and tight intracellular connections that prevent the passive permeability of most drugs. AZ concluded that early screening for brain penetrating drugs generally requires maintaining a molecular weight (MW)< 450, a polar surface area (PSA)< 90 Å2, and a LogD between 1-4, plus more stringent requirements of a PSA< 70 Å2. To further enhance brain penetration, it was suggested that PSA< 90 Å2, HBD< 3, and LogD between 2-5, MW<500. Current guidelines for CNS drug design include PSA< 76 Å2 (preferably within 25 - 60 Å2) and HBD< 3 (ideally 0 or 1). Sotorasib (MW=561, PSA=92 Å2, HBD=1, Log D=2.3), and Adagasib (MW=604, PSA=71 Å2, HBD=0, Log D=3.6) are still far from these ideal features.
AZ has many successful instances of developing drugs for brain metastatic cancer that are now on the market and in clinical trials, including mutant EGFR kinase inhibitor osimertinib (on the market), EGFR kinase inhibitor AZD3759 (undergoing application for market approval), ATM kinase inhibitor AZD1390 (in phase I clinical trials), PARP1 selective inhibitor AZD9574 (in phase I/II clinical trials). In addition, there is DZD1516, a HER2 small molecule inhibitor (in phase I trials), developed by Dizal, which is a joint venture of AstraZeneca.
After multiple rounds of optimization, AZ finally identified the candidate molecule AZD4747, which has a KRAS G12C IC50 of 15 nM, Log D7.4= 2.9, PSA = 53 Å2, HBD = 1, and the molecular weight was also reduced to 441 due to the removal of a pyrimidine ring. AZD4747 exhibits molecular characteristics for drug entry into the CNS system, with low in vitro efflux for human P-gp and BCRP.
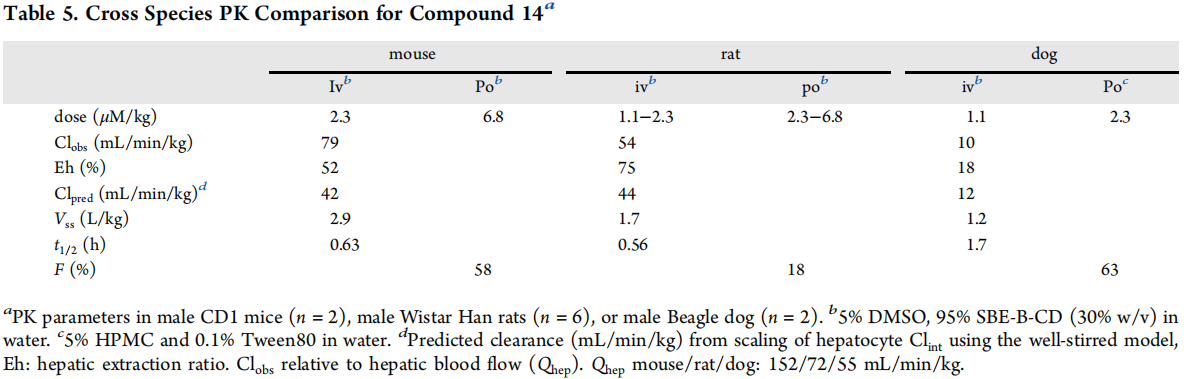
As shown in Figure 3, in rodents, observed clear rates range from medium to high, with liver blood flows in mice and rats at 52% and 75% respectively. The oral bioavailability in mice (58%) was higher than in rats (18%). In dogs, the observed clearance rate was as low as 18% of liver blood flow, with oral bioavailability at 63%. The volume of distribution at steady state across populations was moderate, ranging from 1.7 to 2.9 L/kg. Although the brain Kpu,u obtained in rats was lower than expected at 0.1, the Kpu,u in dogs and monkeys reached the screening criteria at 0.7 and 1.6, respectively.
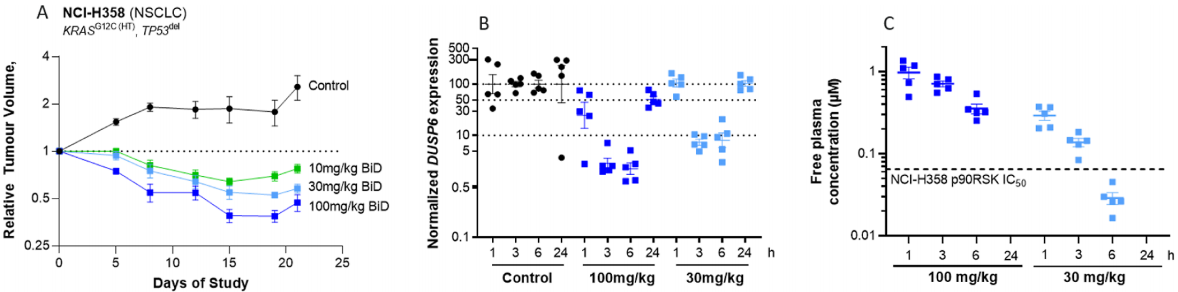
As shown in Figure 4, the therapeutic effect of AZD4747 was tested in an animal model. Mice were treated orally with daily doses of AZD4747 at 100mg/kg, 30mg/kg, and 10mg/kg and demonstrated good tolerance, as well as significant tumor regression at higher doses, in KRAS G12C mutant H358 xenograft tumors. The concentration of AZD4747 in free plasma was linearly related to the dosage. Tumors in mice treated with AZD4747 showed a strong, dose- and exposure-dependent decrease in DUSP6 expression, a factor regulated by the RAS/MAPK pathway.
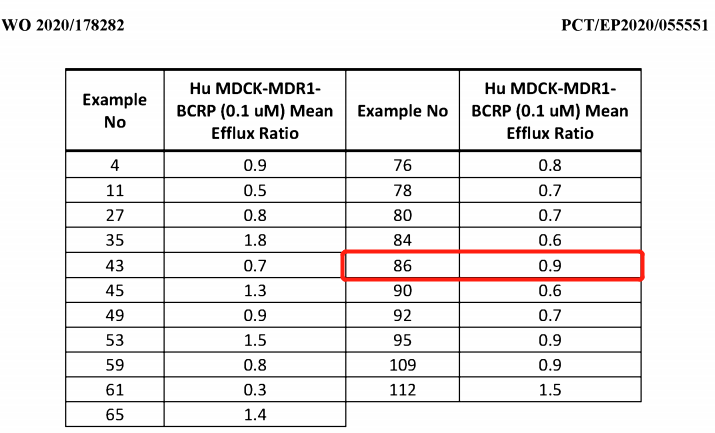
Searching the Patsnap patent database with "KRAS" and "AstraZeneca" as keywords, four patents were primarily found: WO2020178282, WO2019215203, WO2019110751, and WO2018206539. The latter three patents have little to do with KRAS brain penetration inhibitors. Among them, patent WO2020178282, one of examples 85 and 86, is precisely AZD4747; the compounds 85 and 86 are two enantiomeric isomers. Only the activity and excretion data of 86 were tested. 86 is more likely to be AZD4747. The transfected cell line MDCK_MDR1_BCRP expresses two primary excretion pumps that actively exclude the transportation of compounds across the blood-brain barrier. Compounds measured by this method with an excretion ratio of 2 or less are determined to have the potential for excellent blood-brain barrier penetration. All compounds shown in Figure 5 have the potential, and subsequently, compound 86 was determined to have balanced drug potential based on other evaluation data. The earliest priority date of patent WO2020178282 is March 5, 2019; the international application date is March 3, 2020; the international publication date is September 10, 2020; and the domestic open text CN113508118A is expected to expire on March 3, 2040.
Among the two KRAS G12C drugs currently on the market, clinical trials for the published sotorasib research excluded patients with active CNS metastases, so data on CNS permeation in sotorasib is limited. Sabari and colleagues first carried out a retrospective analysis of brain metastases in patients with KRAS-mutant NSCLC, then conducted a comprehensive review of preclinical and pharmacological data for adagrasib. In multiple preclinical encephalomyelitis models, adagrasib penetrated into the cerebrospinal fluid, showing tumor regression and prolonged survival. In two cases of NSCLC and untreated encephalomyelitis, the concentration of adagrasib in the cerebrospinal fluid was higher than the target cell IC50. Both patients showed corresponding brain metastases, supporting potential clinical activity of adagrasib in the brain. This data from only two brain metastasis patients has been criticized. Primarily because the treatment and prevention of brain metastases are crucial for patient prognosis.
These results support further development of adagrasib in patients with untreated brain metastases and KRAS G12C-mutant NSCLC (NCT03785249). This phase I/II clinical trial enrolled 822 patients, evaluating the safety, tolerance, drug levels, molecular effects and clinical activity of MRTX849 (adagrasib) in patients with late-stage solid tumors with a KRAS G12C mutation. It is expected to be completed in September 2024.
Additionally, in clinical trials, D-1553 developed by Benefit Bio has found that one fortunate patient achieved partial remission in three patients with baseline brain metastases, with an overall objective remission rate of 17%. Before treatment, the size of the brain metastases exceeded 20mm, after treatment the tumor lesions reduced by nearly half, and two patients remained stable, achieving a disease control rate of 100%.
Cancer patients with brain metastases are likely to have CNS damage and impairment of the blood-brain barrier function, allowing compounds with no brain access in preclinical animal model evaluations to have brain-penetrating effects in clinical trials. AZ has experience in successfully developing several brain-penetrating drugs – their further research is expected to benefit clinical patients.

References
1.Oliver Steward et.al; Discovery of AZD4747, a Potent and Selective Inhibitor of Mutant GTPase KRASG12C with Demonstrable CNS Penetration.doi.org/10.1021/acs.jmedchem.3c00746.
2.Nagasaka et al;Penetrating the central nervous system sanctuary of KRAS, a target once thought “undruggable”.Transl Lung Cancer Res 2023;12(4):665-668.
3.WO2020178282
4.CN113508118A
5.https://clinicaltrials.gov/study/NCT03785249?term=NCT03785249&rank=1.