Edgewise Therapeutics Announces Positive Phase 1 and Phase 2 CIRRUS-HCM Study Results in Obstructive Hypertrophic Cardiomyopathy
Edgewise Therapeutics, Inc. (Nasdaq: EWTX), a prominent biopharmaceutical firm specializing in muscle diseases, announced the primary results for EDG-7500 from its Phase 1 clinical study involving healthy participants and the single-dose segment of the Phase 2 CIRRUS-HCM trial targeting obstructive HCM patients. EDG-7500 is a new oral, selective modulator of cardiac sarcomeres, developed to decelerate early contraction speed and ameliorate the impaired cardiac relaxation characteristic of HCM.
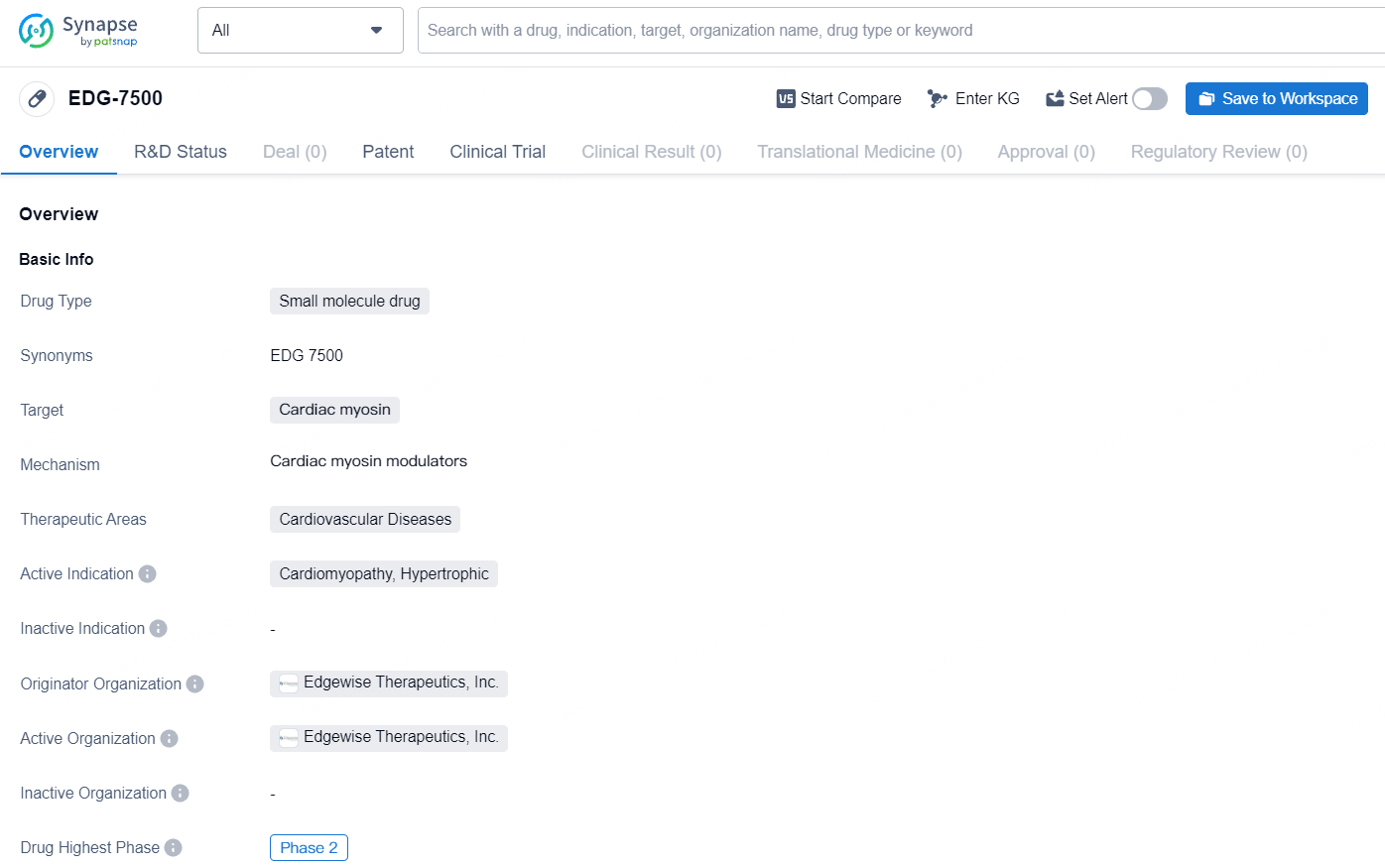
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
In the placebo-controlled Phase 1 single ascending dose (SAD) study (n=48), healthy participants received single doses of EDG-7500, varying from 5 to 300 mg. During the multiple ascending dose (MAD) phase of the study (n=24), participants were administered 25 to 100 mg daily over a period of 14 days. Both the SAD and MAD trials showed EDG-7500 was well tolerated, with no significant changes or trends detected in vital signs, clinical chemistry, hematology, or ECGs. Additionally, left ventricular ejection fraction (LVEF) remained stable across all subjects in both the SAD and MAD trials regardless of the dose range. The MAD trial identified a half-life of around 30 hours, reaching steady state within approximately 4 days of daily dosing. Furthermore, exposure levels in the SAD and MAD phases increased in a manner that was generally dose-proportional.
In CIRRUS-HCM Part A, patients with obstructive hypertrophic cardiomyopathy (HCM) received single doses of 50, 100, or 200 mg of EDG-7500. Those receiving 100 and 200 mg doses experienced a 67% mean reduction in resting left ventricular outflow tract pressure gradient (LVOT-G) and a 55% mean reduction in Valsalva-induced LVOT-G. A reduction in LVOT gradients to less than 30 mmHg at rest and less than 50 mmHg during Valsalva was seen in 60% of patients on the 100 or 200 mg doses of EDG-7500. Notably, this decrease was achieved without a significant change in LVEF. A single dose of 200 mg EDG-7500 also resulted in a 64% mean reduction in NT-proBNP, a critical heart failure biomarker, emphasizing the therapeutic potential for treating diastolic dysfunction diseases, including non-obstructive HCM.
Throughout the Phase 1 and CIRRUS-HCM studies, no subjects exhibited a reduction in LVEF below 50% across different EDG-7500 exposures.
"In light of the robust clinical and preclinical data thus far, we have commenced the 28-day portion of CIRRUS-HCM in patients with both obstructive and non-obstructive HCM," said Marc Semigran, M.D., Chief Development Officer at Edgewise Therapeutics. "We will continue our assessment of tolerability, pharmacokinetics, and effects on LVOT-G, LVEF, biomarkers, and patient-reported outcomes in these cohorts."
Dr. Anjali T. Owens, Medical Director at the Center for Inherited Cardiac Disease and Associate Professor of Medicine at the University of Pennsylvania, remarked, "There remains a significant unmet need for therapies in both obstructive and non-obstructive HCM, and we are enthusiastic about our involvement in the ongoing CIRRUS-HCM trial of this novel treatment."
Kevin Koch, Ph.D., President and Chief Executive Officer at Edgewise Therapeutics, added, "Our innovative strategy, demonstrating gradient relief without LVEF reduction, holds promise for advancing the treatment of obstructive HCM. We anticipate releasing initial 28-day data in Q1 2025."
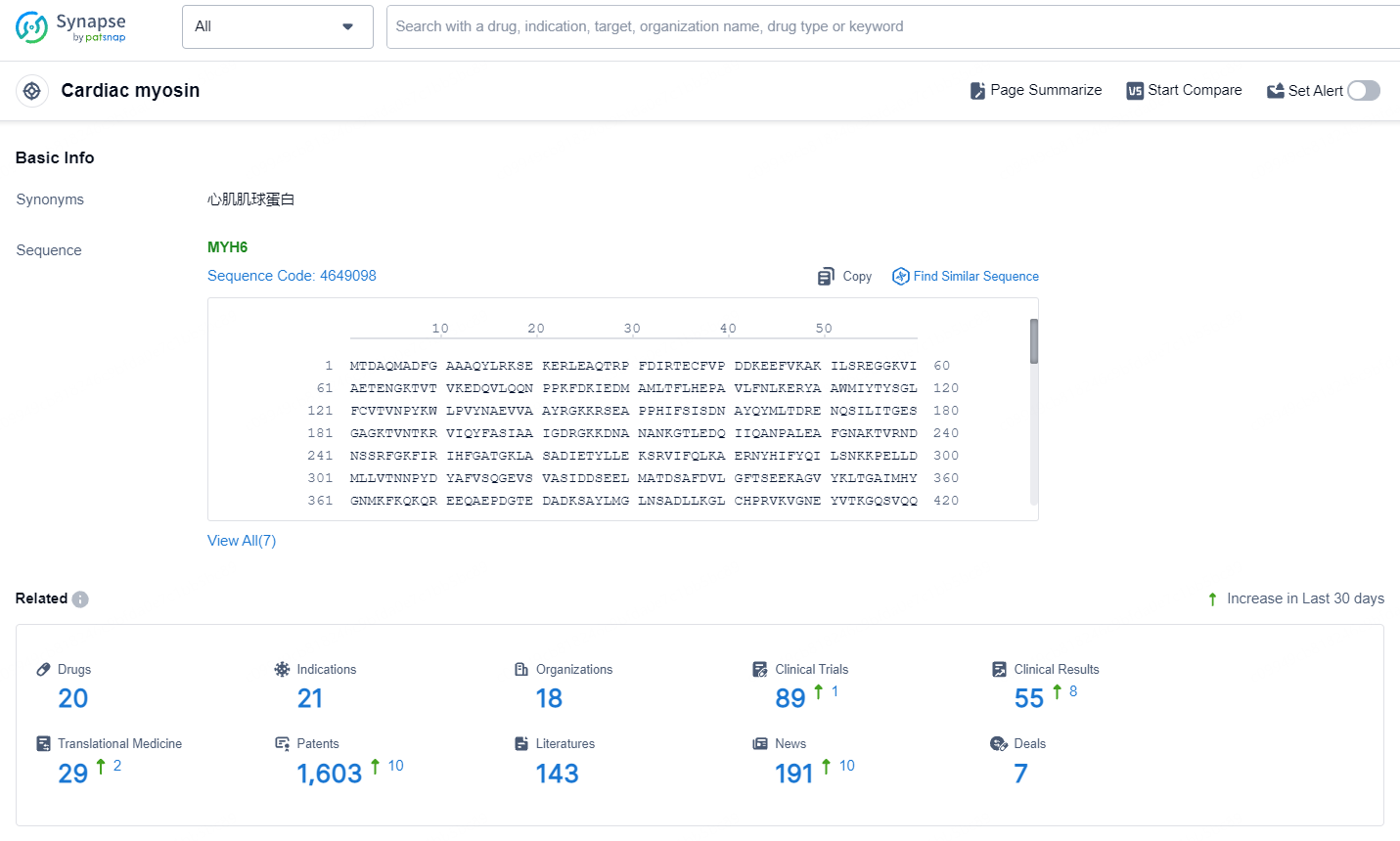
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 23, 2024, there are 20 investigational drugs for the cardiac myosin targets, including 21 indications, 18 R&D institution involved, with related clinical trial reaching 89, and as many as 1603 patents.
EDG-7500 is a small molecule drug developed by Edgewise Therapeutics, Inc., targeting cardiac myosin for the treatment of cardiovascular diseases, specifically cardiomyopathy and hypertrophic cardiomyopathy. It is currently in the highest phase of clinical development, which is Phase 2. As a small molecule drug, EDG-7500 is designed to act at the molecular level to modulate the function of cardiac myosin, a protein that plays a crucial role in heart muscle contraction. By targeting this specific protein, the drug aims to address the underlying mechanisms of cardiovascular diseases, particularly cardiomyopathy and hypertrophic cardiomyopathy.