Elicio Therapeutics Reports Initial Participant Receives Treatment in Phase 2 Trial for Pancreatic Cancer
Elicio Therapeutics, Inc., an enterprise at the clinical trial phase that focuses on creating a range of innovative immunotherapies aimed at combating cancer, revealed that the inaugural participant has received treatment within the context of a Phase 2 controlled study. This study is evaluating ELI-002 7P as a standalone adjuvant therapy in patients with KRAS mutated pancreatic ductal adenocarcinoma. The dosing occurred at the Northwell Health Cancer Institute and the Feinstein Institutes for Medical Research located in New York.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
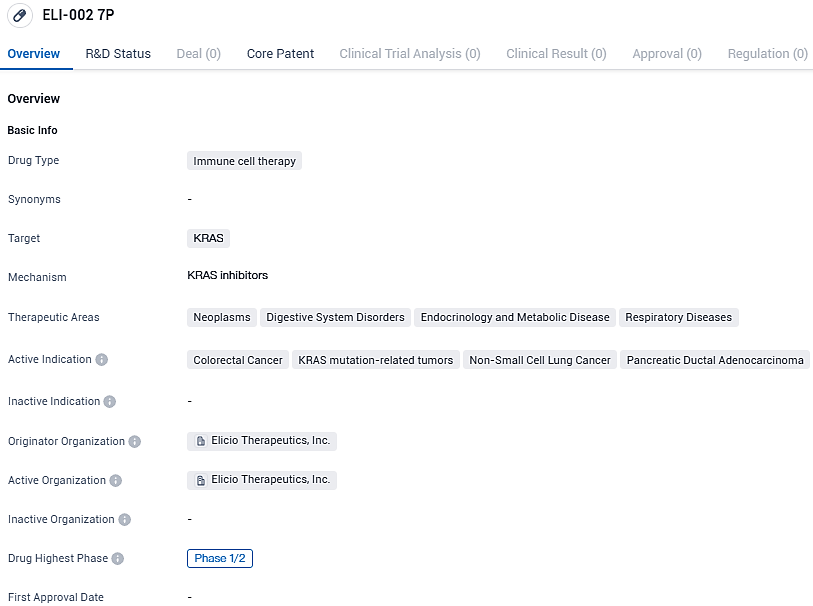
ELI-002 7P is a novel cancer immunotherapy currently under investigation, created using Elicio’s unique Amphiphile technology aimed specifically at the lymph nodes. It's designed to combat a selection of malignant tumors that are driven by a set of seven prevalent KRAS mutations found in one-quarter of all solid tumors and the vast majority (88%) of patients with PDAC.
In contrast, other treatments pursuing KRAS mutations – predominantly those small molecule inhibitors – have a more narrow mutation target scope, which may restrict the treatment applicability to a smaller patient population and may also lead patients to develop resistant mutant cells, decreasing the long-term benefits.
"ELI-002 is an innovative vaccination strategy targeting a wider range of KRAS mutations specific to pancreatic cancer. The Phase 2 clinical trial is based on promising results from earlier research using a 2-peptide version of ELI-002 that was featured in Nature Medicine, showing a significant reduction in cancer markers," said Christopher Haqq, M.D., Ph.D., and Executive Vice President at Elicio. "We are hopeful that the outcomes of this trial will provide conclusive evidence to evaluate its clinical effectiveness."
At the upcoming ASCO Gastrointestinal Cancers Symposium, scheduled for January 18-20, 2024, in San Francisco, CA, Elicio is set to showcase a detailed poster about the ongoing AMPLIFY-7P trial. Preliminary phase 1A results of the ELI-002 7P as a single-agent treatment are expected to be released in the first half of 2024.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
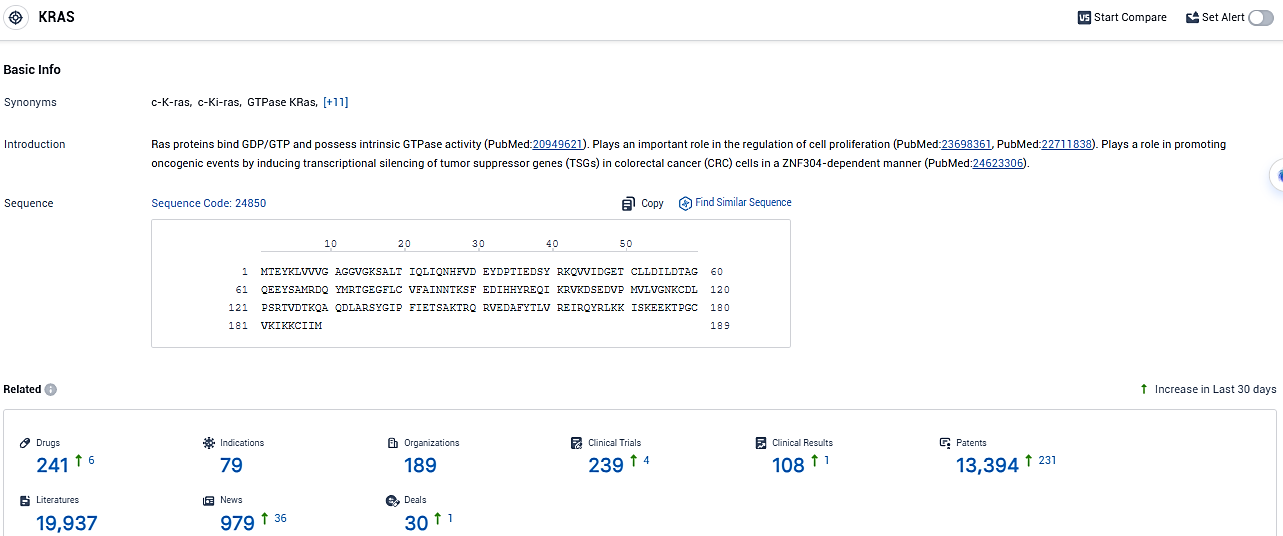
According to the data provided by the Synapse Database, As of January 16, 2024, there are 241 investigational drugs for the KRAS target, including 79 indications, 189 R&D institutions involved, with related clinical trials reaching 239, and as many as 13394 patents.
ELI-002 7P targets KRAS and aims to address various types of cancers, including colorectal cancer, KRAS mutation-related tumors, non-small cell lung cancer, and pancreatic ductal adenocarcinoma. The drug is currently in Phase 1/2, indicating its progress in the development pipeline.