Eton Pharmaceuticals, Inc plans to purchase Increlex® (mecasermin injection) from Ipsen
Eton Pharmaceuticals, Inc (Nasdaq: ETON), a forward-thinking pharmaceutical firm specializing in the development and commercialization of therapies for rare conditions, revealed that it has engaged in an asset purchase deal to obtain Increlex® (mecasermin injection) from Ipsen S.A. The transaction is anticipated to finalize around the end of 2024.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Eton's mission focuses on creating and distributing medications with transformative impacts for individuals dealing with extremely rare health conditions. Increlex, a vital drug for those suffering from the ultra-rare severe IGF-1 deficiency, is perfectly aligned with this mission and our deep proficiency in addressing the needs of highly rare patient groups," stated Sean Brynjelsen, CEO of Eton Pharmaceuticals. "By leveraging our robust position within the pediatric endocrinology community, we strive to enhance awareness of this often overlooked and inadequately treated condition. We are eager to collaborate with Ipsen to guarantee uninterrupted care and a reliable long-term supply for patients around the world."
Increlex is a biologic therapy designed for children and teenagers aged 2 to 18 who experience severe primary insulin-like growth factor 1 deficiency (SPIGFD) due to insufficient production of insulin-like growth factor 1 (IGF-1) in their bodies. The drug has received approval in 40 regions, including the United States (U.S.) and the European Union (EU). It is estimated that about 200 patients in the U.S. and 900-1,000 patients in Europe are affected by SPIGFD. Increlex is the sole treatment endorsed by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for SPIGFD.
Upon closure of the deal, Eton will take over the product’s commercialization in the U.S. without interrupting patient access. Outside the U.S., Ipsen will maintain product distribution for a six-month transition period to ensure continued patient supply, after which Eton will take over the commercialization process.
The acquisition will be funded through Eton’s existing cash reserves and an expansion of its current credit agreement with SWK Holdings. Ipsen reported worldwide sales of Increlex totaling €17.3 million in 2023.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
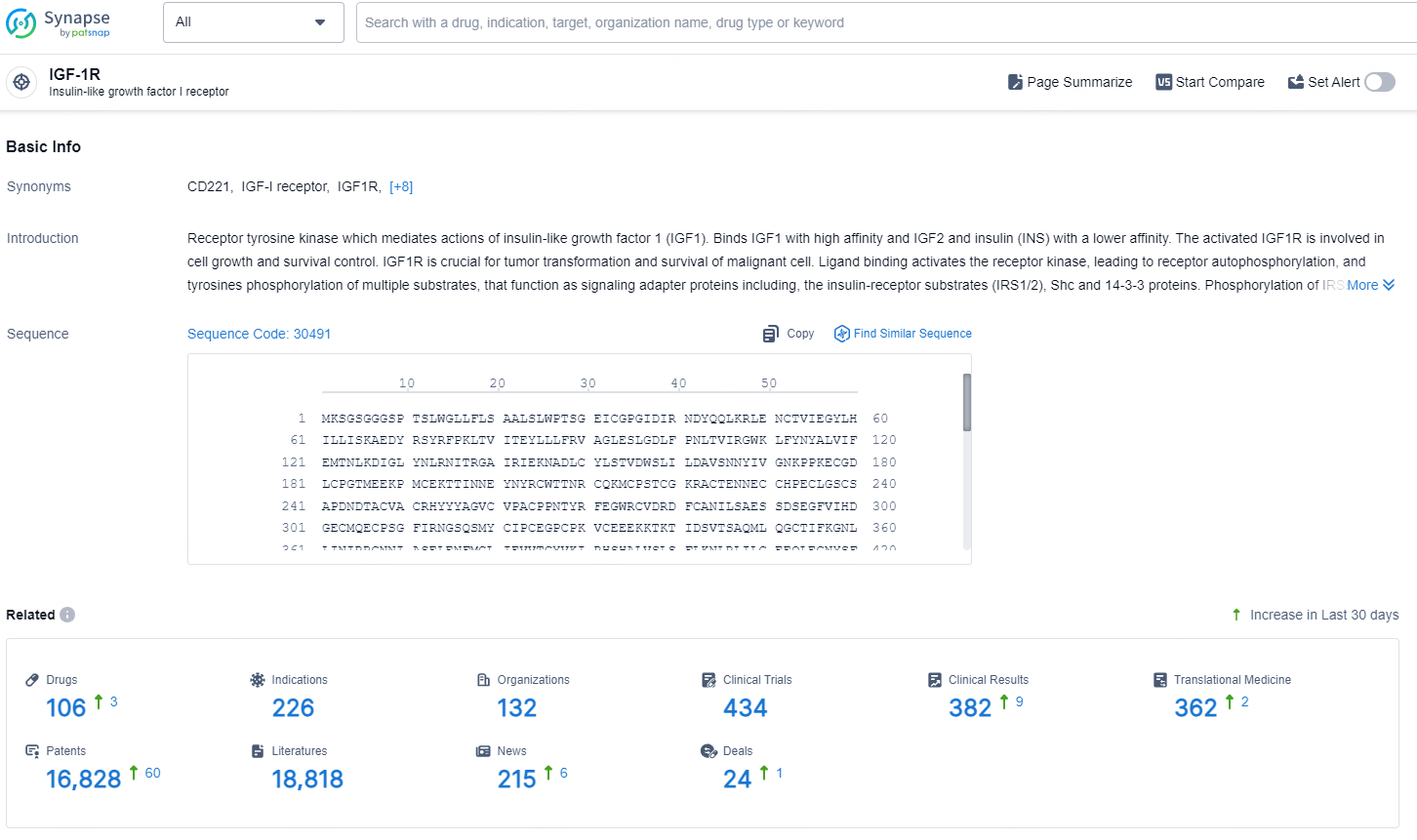
According to the data provided by the Synapse Database, As of October 9, 2024, there are 106 investigational drugs for the IGF-1R targets, including 226 indications, 132 R&D institutions involved, with related clinical trials reaching 434, and as many as 16828 patents.
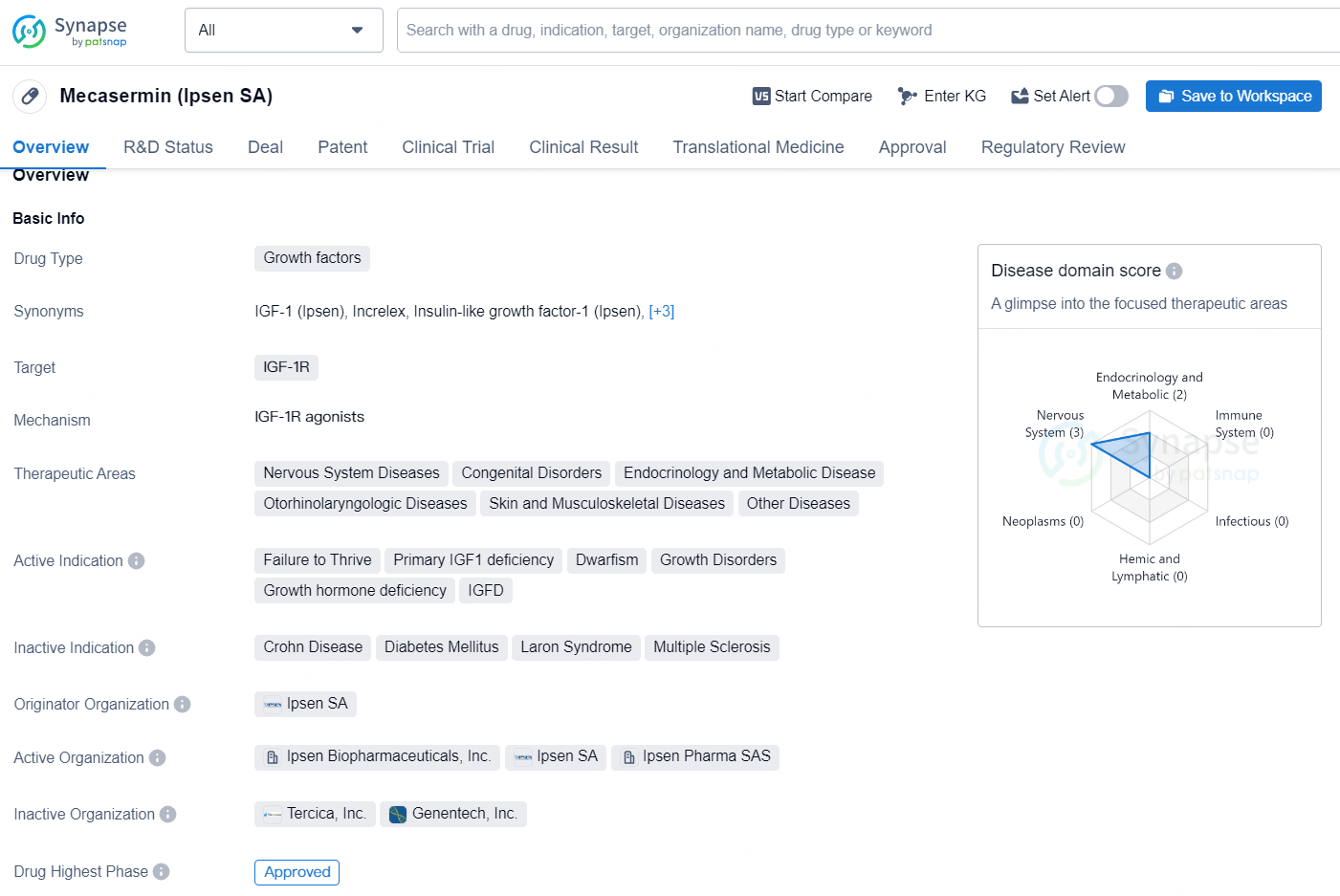
Mecasermin (Ipsen SA) is a drug classified as a growth factor, with its main targets being IGF-1R. Its therapeutic areas include Nervous System Diseases, Congenital Disorders, Endocrinology and Metabolic Disease, Otorhinolaryngologic Diseases, Skin and Musculoskeletal Diseases, as well as other diseases. The drug is specifically indicated for the treatment of Failure to Thrive, Primary IGF1 deficiency, Dwarfism, Growth Disorders, Growth hormone deficiency, and IGFD.