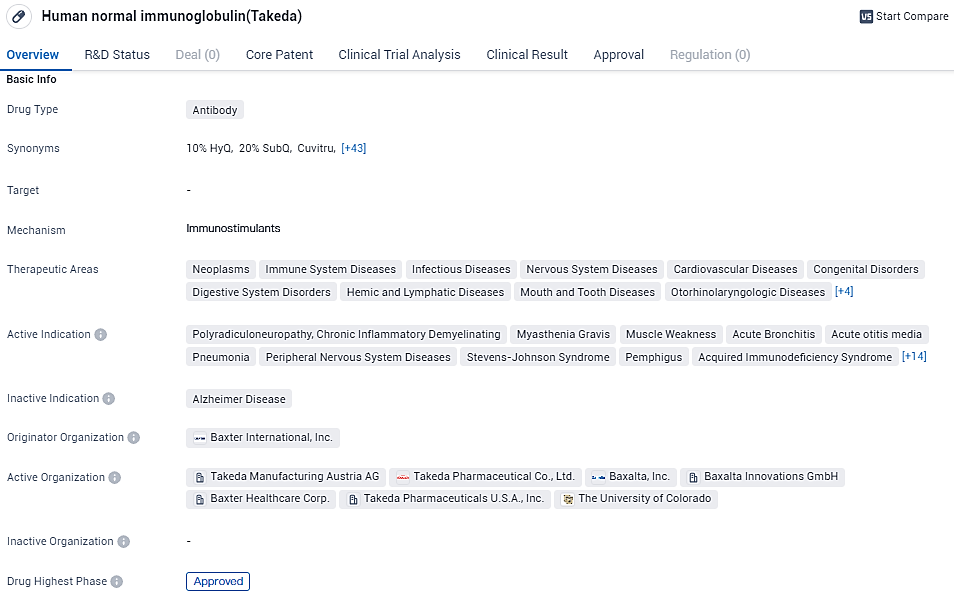
EU Commission Approves Takeda's HYQVIA® for Chronic Inflammatory Demyelinating Polyneuropathy Treatment
Takeda has disclosed that the European Commission has sanctioned the use of HYQVIA® [Immune Globulin Infusion 10% with Recombinant Human Hyaluronidase] for long-term treatment across all age groups afflicted with chronic inflammatory demyelinating polyneuropathy, contingent on initial patient condition stabilization achieved through intravenous immunoglobulin therapy.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Takeda had previously disclosed a favorable assessment from the Committee for Medicinal Products for Human Use on December 15, 2023, and the green light for its use as a continuation treatment in adult patients suffering from CIDP was granted by the U.S. Food and Drug Administration on January 16, 2024.
HYQVIA, representing the only product of its kind to enable facilitated subcutaneous delivery of immunoglobulin for patients with CIDP, provides a means for up to once-per-month infusion. Through the action of the hyaluronidase component, it aids the spread and uptake of high volumes of immunoglobulin beneath the skin but above the muscle tissue. Patients, after receiving adequate instruction, may either self-administer HYQVIA or have it administered by a healthcare provider in their residence.
Kristina Allikmets, the leader of Research & Development at Takeda’s Plasma-Derived Therapies Business Unit and a high-ranking executive, commented that the endorsement by the European Commission for HYQVIA in managing CIDP marks an essential milestone. It ensures that individuals throughout Europe with CIDP have the opportunity to benefit from a reliably effective maintenance treatment that can be facilitated monthly either at their abode or clinically.
Allikmets further noted that the broadening of HYQVIA's indications is a testament to Takeda's dedication to advancing their immunoglobulin treatments for those affected by neuroimmunological conditions, aiming to introduce treatment alternatives capable of significantly improving patient outcomes and raising the bar for healthcare standards.
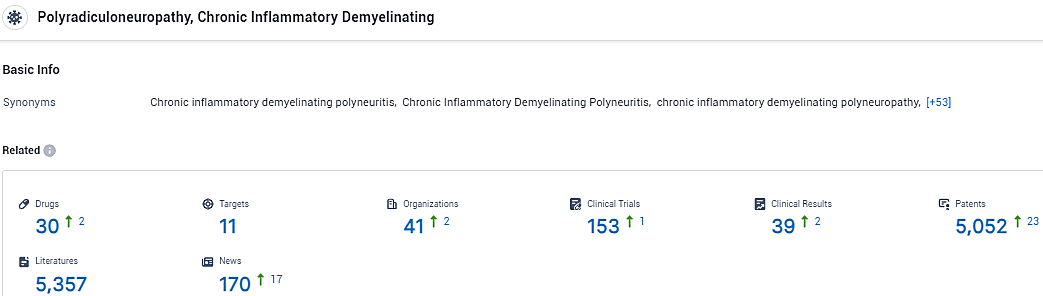
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
According to the data provided by the Synapse Database, As of February 2, 2024, there are 30 investigational drugs for the Chronic inflammatory demyelinating polyneuritis, including 11 targets, 41 R&D institutions involved, with related clinical trials reaching 153, and as many as 5052 patents.
HYQVIA is a significant drug in the pharmaceutical industry, offering therapeutic benefits to patients with diverse medical conditions. Its approval history, wide range of indications, and originator organization contribute to its credibility and importance in the field of biomedicine.