Exploring Empagliflozin: Therapeutic Uses and Advances in Pharmaceutical Development
Empagliflozin is a medication used primarily to treat type 2 diabetes and, in some cases, to help manage heart failure. It belongs to a class of drugs known as sodium-glucose co-transporter 2 (SGLT2) inhibitors. These medications work by preventing the kidneys from reabsorbing glucose back into the blood. Instead, they help the body excrete excess glucose through urine, thereby lowering blood sugar levels.
In addition to its glucose-lowering effects, empagliflozin has shown benefits in reducing the risk of cardiovascular disease, particularly in patients with diabetes who have existing heart disease. It might also help with weight loss and blood pressure reduction.
Empagliflozin is often used in conjunction with other diabetes medications and lifestyle modifications like diet and exercise to manage blood sugar levels effectively. As with any medication, it is important to use empagliflozin under the guidance of a healthcare provider, as it can have potential side effects and interactions with other medications.
Empagliflozin was first approved globally in April 2014 and was initially approved in Australia. It is originated by Boehringer Ingelheim GmbH. The drug has obtained the highest phase of approval in both the global and Chinese markets.
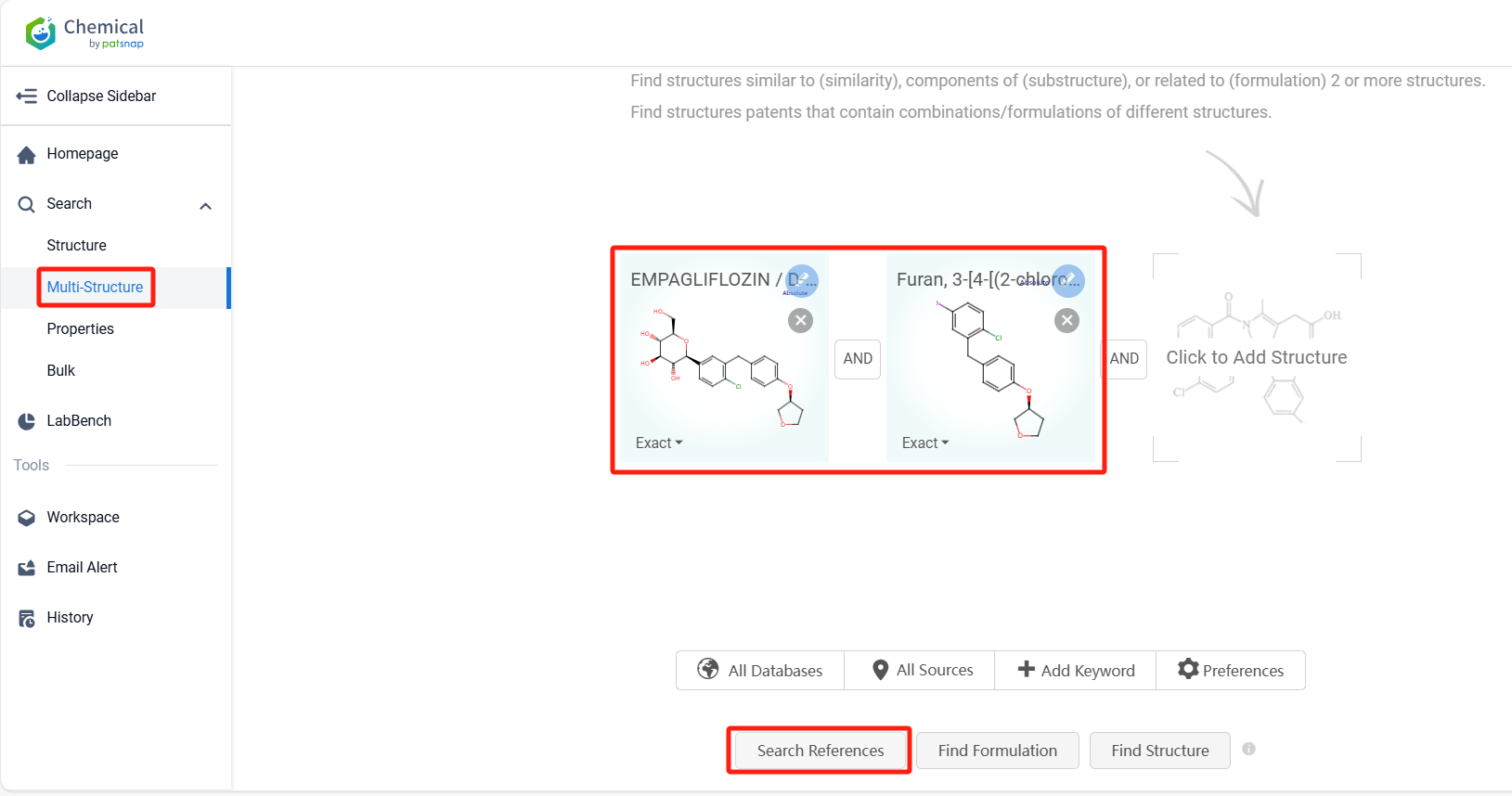
Log in to the Patsnap Chemical. Select the Multi-Structure search, and search for the reactants and products in the specific steps of the synthesis route of Empagliflozin. click on search references, and you can query the relevant literature.
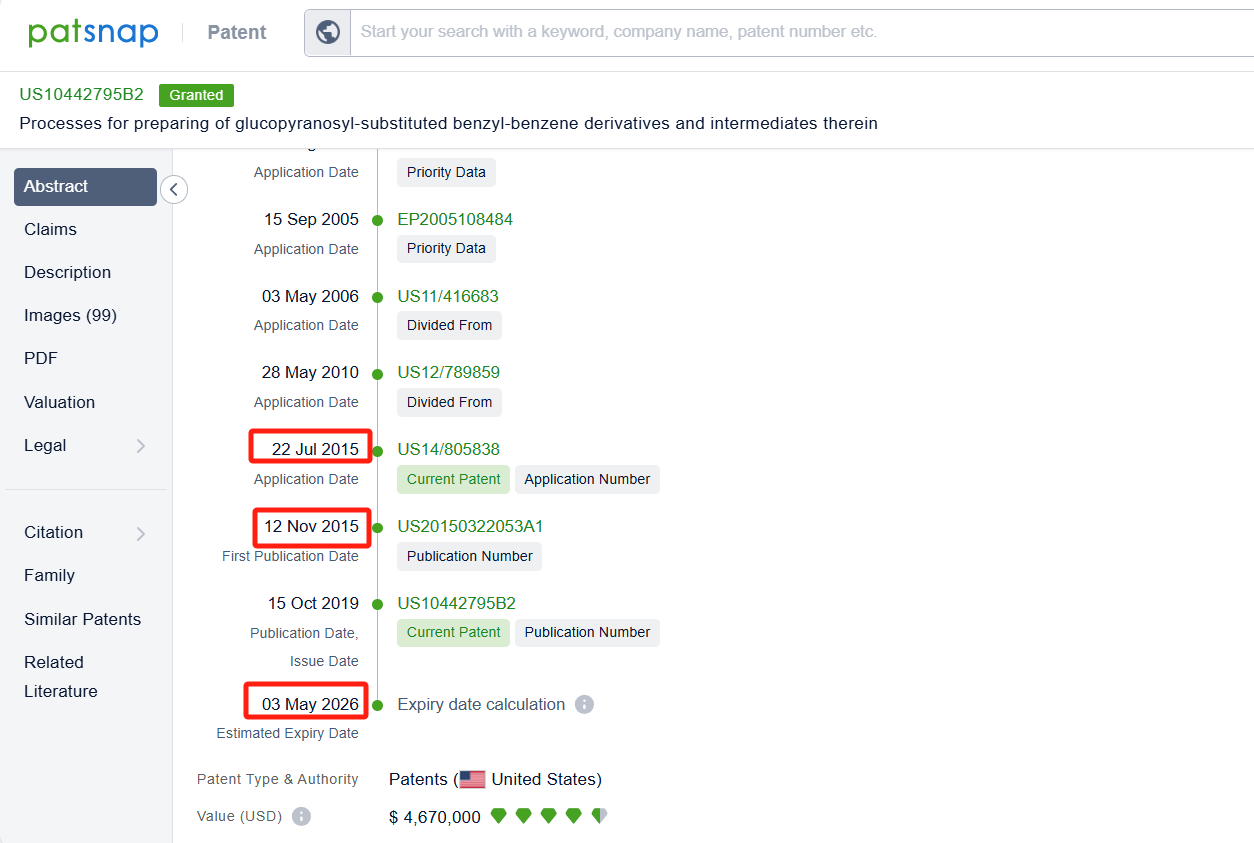
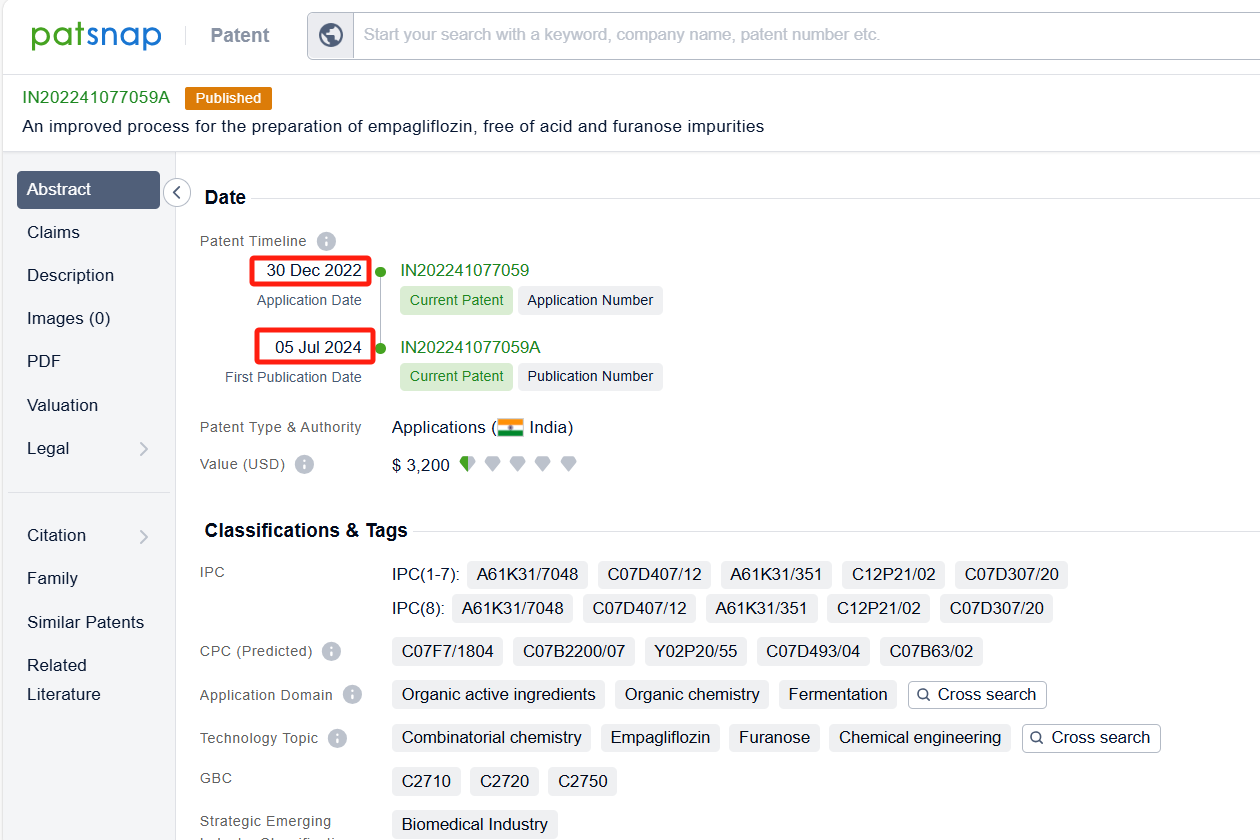
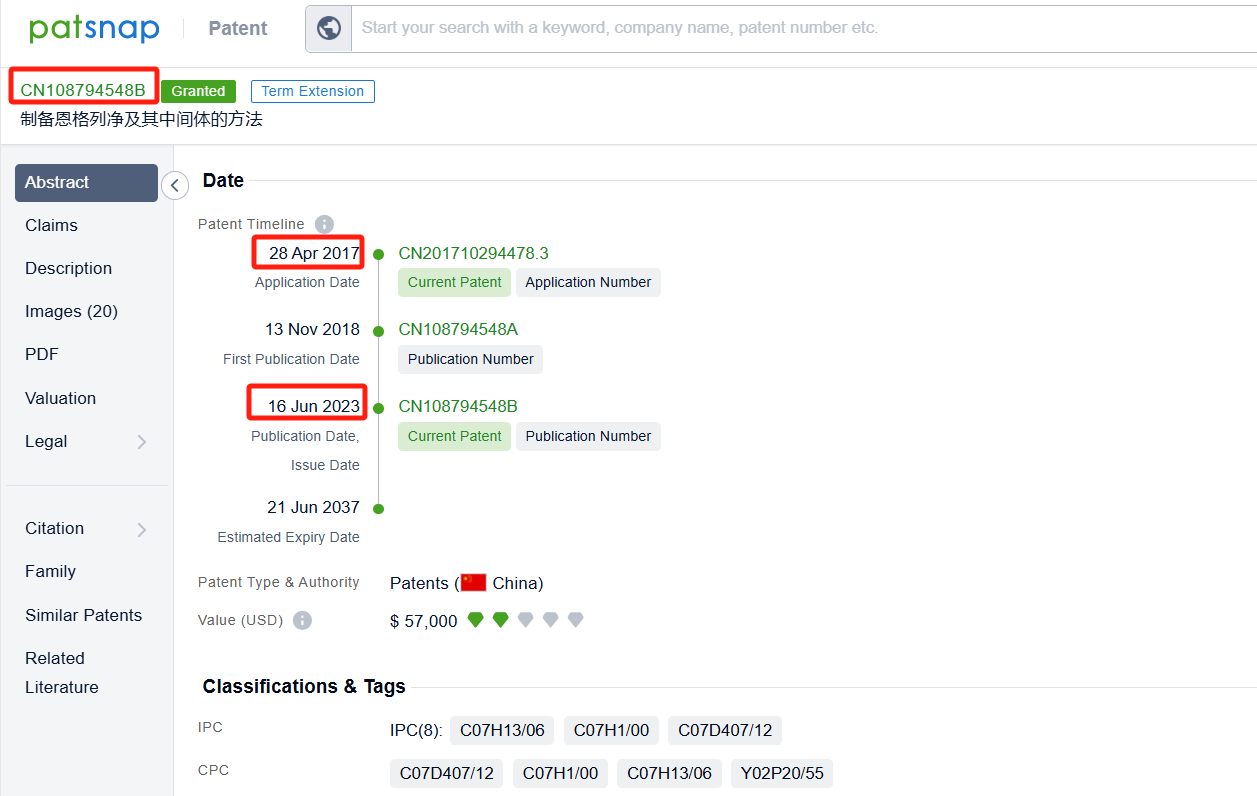
 Linked to the Patsnap patent, check the 'Chemistry' category under the technical subject classification and click on the filter, which will allow you to accurately retrieve the design and improvement of the Empagliflozin process route by Boehringer Ingelheim International GmbH and other companies,Such as Boehringer Ingelheim International GmbH's patent US10442795B2 (application date 20150722, publication date 20151112) relates to a process for preparing glucopyranosyl-substituted benzyl-benzene derivatives, The process allows for the production of these compounds in high yields, purity, and with low technical expenditure. The patent was granted on October 15, 2019, with the patent expiration date being May 3, 2026. Granules India Ltd.'s patent IN202241077059A (application date 20221230, publication date 20240705) describes an improved process for the preparation of empagliflozin, free of acid and furanose impurities. Additionally, Chia Tai Tianqing Pharmaceutical Group Co., Ltd.'s patent CN108794548B (application date 20170428, publication date 20181113) describes a method for preparing empagliflozin and its intermediate. Its corresponding patents in Japan, South Korea, and the United States have all been granted. The patent was granted on June 16, 2023, with an expiration date of June 21, 2037.
Linked to the Patsnap patent, check the 'Chemistry' category under the technical subject classification and click on the filter, which will allow you to accurately retrieve the design and improvement of the Empagliflozin process route by Boehringer Ingelheim International GmbH and other companies,Such as Boehringer Ingelheim International GmbH's patent US10442795B2 (application date 20150722, publication date 20151112) relates to a process for preparing glucopyranosyl-substituted benzyl-benzene derivatives, The process allows for the production of these compounds in high yields, purity, and with low technical expenditure. The patent was granted on October 15, 2019, with the patent expiration date being May 3, 2026. Granules India Ltd.'s patent IN202241077059A (application date 20221230, publication date 20240705) describes an improved process for the preparation of empagliflozin, free of acid and furanose impurities. Additionally, Chia Tai Tianqing Pharmaceutical Group Co., Ltd.'s patent CN108794548B (application date 20170428, publication date 20181113) describes a method for preparing empagliflozin and its intermediate. Its corresponding patents in Japan, South Korea, and the United States have all been granted. The patent was granted on June 16, 2023, with an expiration date of June 21, 2037.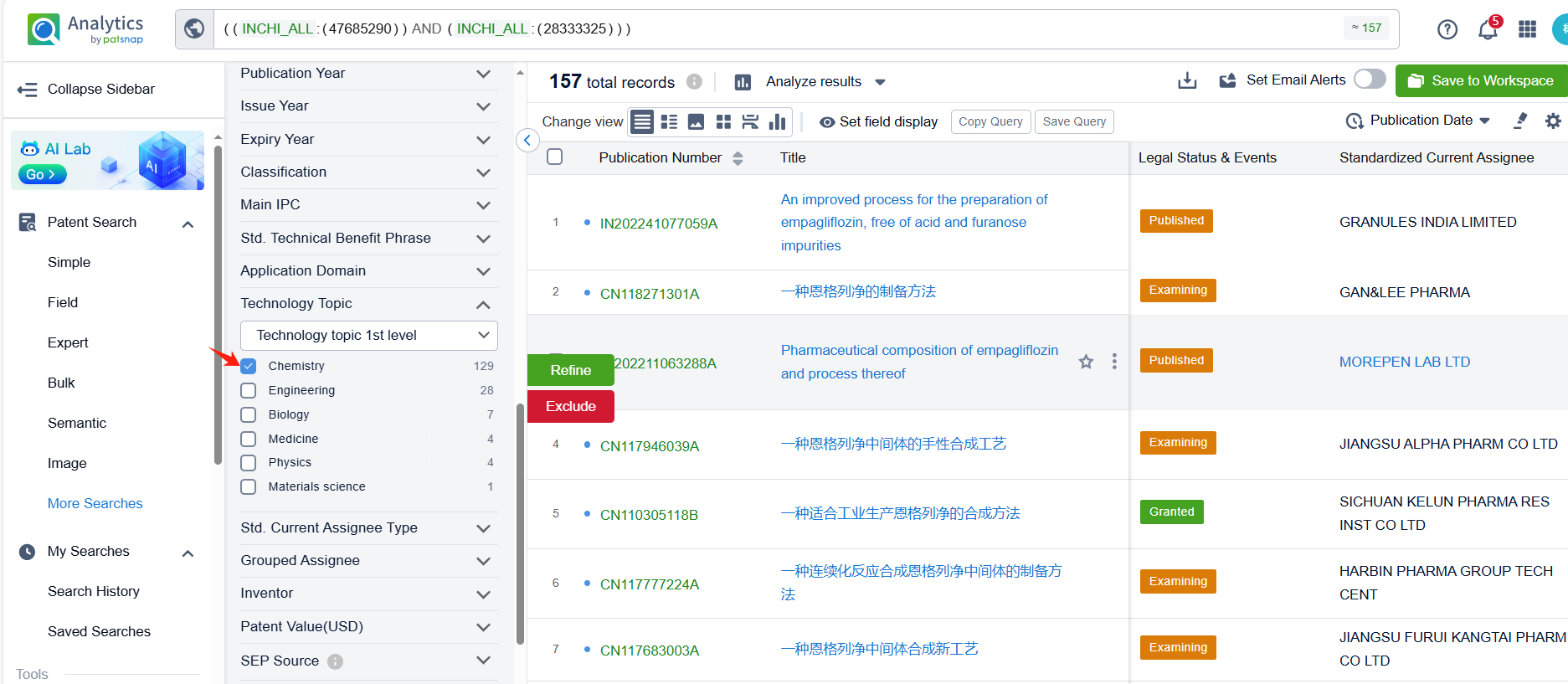
Overall, Empagliflozin is a versatile drug used in a wide array of therapeutic areas and has shown efficacy in the treatment of various conditions such as heart failure, kidney diseases, and diabetes mellitus type 2, among others. Its approval in multiple countries and its status as a breakthrough therapy indicate its potential to significantly impact the pharmaceutical industry and make a positive contribution to patient care. As a small molecule drug, it serves as an important therapeutic option for patients and healthcare providers worldwide, and its approval in both global and Chinese markets further highlights its significance in the field of biomedicine.
AI built to maximize IP and R&D efficiency
Redefine chemical FTO with a range of structure retrieval options at your fingertips, from exact matches to similarity searches, all powered by deep data processing techniques and proprietary AI algorithms to eliminate the risk of omitting key results.




