Exploring the Latest AR PROTAC Deal by Arvinas: A Guide to Rapidly Accessing Transaction Insights
On April 12, 2024, Arvinas announced that it has entered into an exclusive strategic license agreement with Novartis to collaborate on the development of Arvinas' second-generation androgen receptor (AR) degrader, ARV-766, for use in patients with prostate cancer, and that Arvinas is selling another Arvinas has also sold AR-V7, a preclinical, investigational, targeted AR degradation agent, to Novartis.
Under the terms of the agreement, Novartis will be responsible for the global clinical development and commercialization of ARV-766 and will own all research, development, manufacturing and commercialization rights to the preclinical AR-V7 program; Arvinas will receive an upfront payment of $150 million and will be eligible to receive up to $1.01 billion in additional development, regulatory and commercial milestones, as well as tiered royalties on ARV-766. royalties.
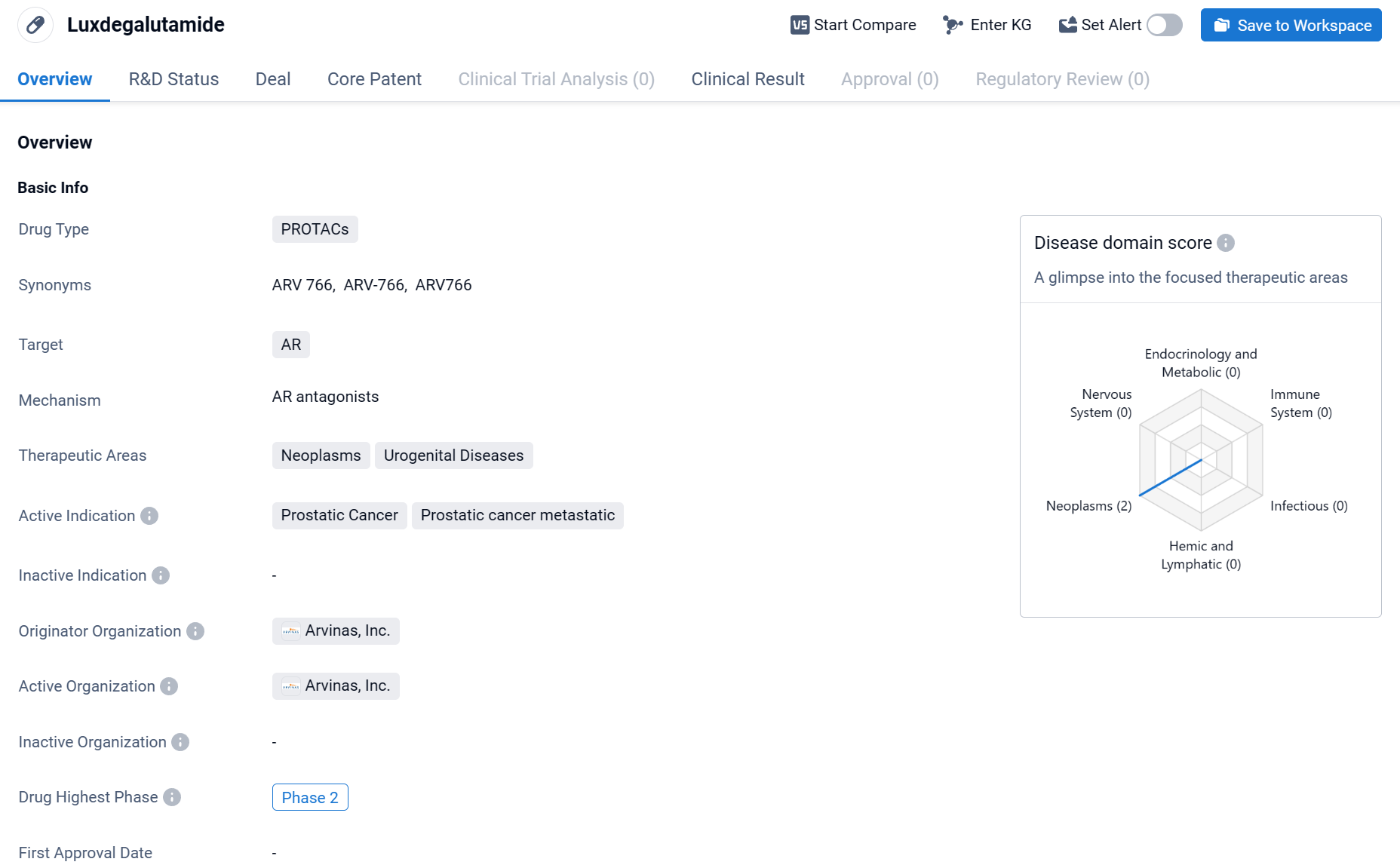
About Luxdegalutamide
Luxdegalutamide (ARV-766) is a drug classified as PROTACs, which stands for Proteolysis Targeting Chimeras. This type of drug works by utilizing small molecules to target specific proteins for degradation within cells. In the case of Luxdegalutamide, its target is the androgen receptor (AR). The therapeutic areas that Luxdegalutamide focuses on are neoplasms and urogenital diseases. Click the image below to directly embark on the exploration journey with the ARV-766!
Compared to ARV-110, the first-generation AR PROTAC-targeting drug developed by the Company, ARV-766 is able to achieve coverage of the AR L702H mutation more efficiently while having degradation capabilities for a variety of mutant subtypes such as H875Y and T878A; animal models have demonstrated ARV-766's degradation strength and tumor antiproliferative activity.
Previously, interim data from the Company's Phase 1/2 dose-escalation and extension trial for the treatment of men with metastatic desmoplasia-resistant prostate cancer (mCRPC), disclosed at 2023 ESMO, demonstrated that ARV-766 demonstrated broad efficacy and excellent tolerability in mCRPC patients harboring AR LBD mutations, including AR L702H.
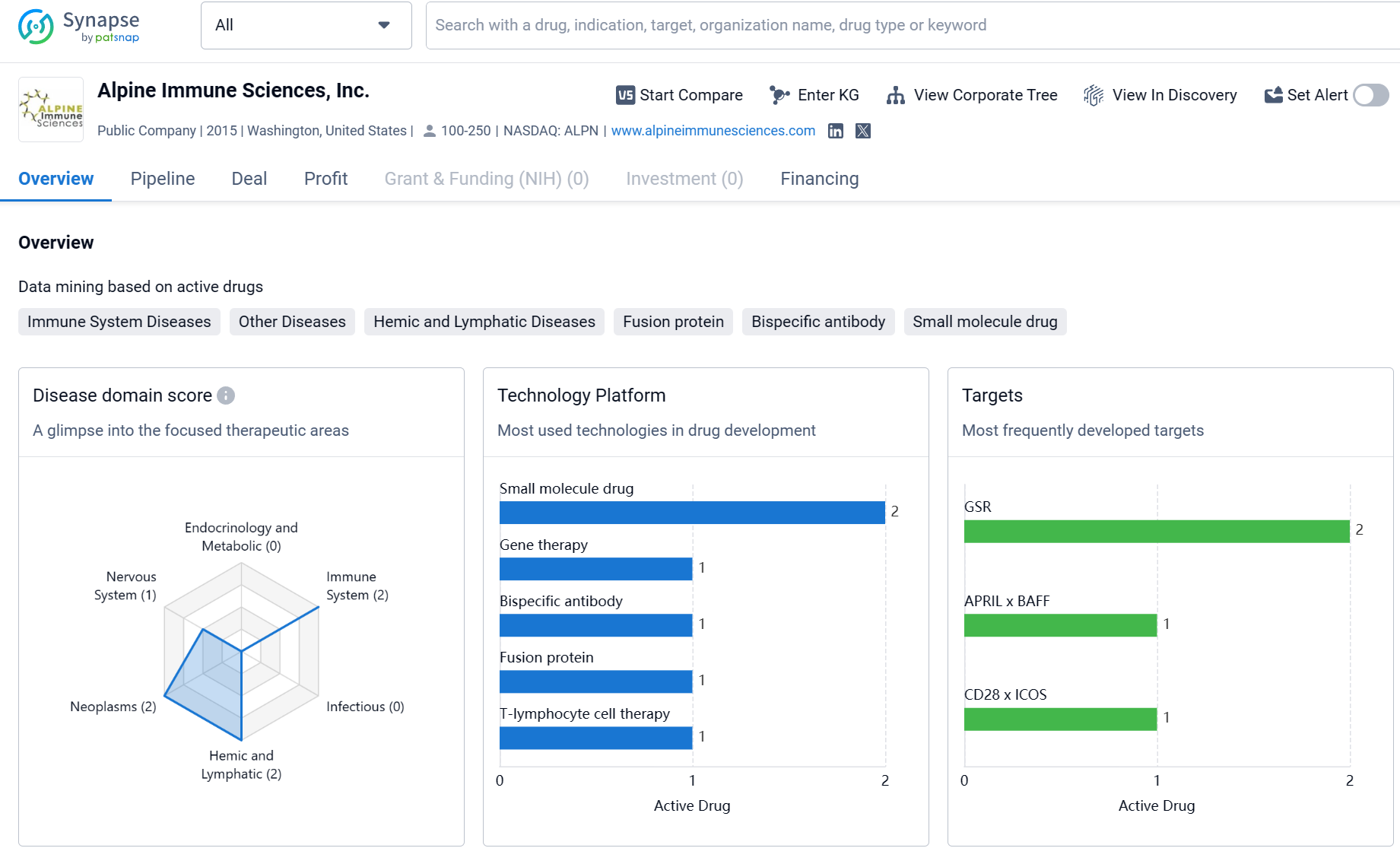
About Arvinas
Arvinas, Inc. is a biopharmaceutical company that focuses on developing innovative therapies in the field of biomedicine. The company has a diverse portfolio of drugs targeting various therapeutic areas, with a strong emphasis on Neoplasms. The most frequently developed targets by Arvinas, Inc. include AR, BRD4, AR-v7, LRRK2, ERα, KRAS G12D x KRAS G12V, SMARCA2, and BCL6. These targets represent a wide range of diseases and conditions, indicating the company's commitment to addressing unmet medical needs. Arvinas, Inc.'s pipeline consists of drugs in different stages of development, with a significant number in the preclinical phase. The company has one drug that has received IND approval and is currently in Phase 1, indicating progress in advancing its therapies towards clinical trials. Overall, Arvinas, Inc. shows promise in its efforts to develop innovative therapies in the biomedicine field.
The Company has four clinical-stage programs in development: vepdegestrant (ARV-471) for the treatment of patients with locally advanced or metastatic ER+/HER2- breast cancer; ARV-766 and bavdegalutamide for the treatment of men with metastatic desmoplasia-resistant prostate cancer; and ARV-102 for the treatment of patients with neurodegenerative disease patients. Recently, the Company disclosed at 2024 AACR the latest research progress of ARV-393, a targeted BCL6 degrader, which has now completed studies related to the submission of an Investigational New Drug (IND) application.
How to get the latest progress on drug deals?
If you would like to access the latest transaction event information, you can click on the 'Deal' module from the homepage of the Synapse database. Within the Deal module, you can search for global pharmaceutical transaction information using labels such as Drugs, Organization, Target, Drug Type, Deal Date.
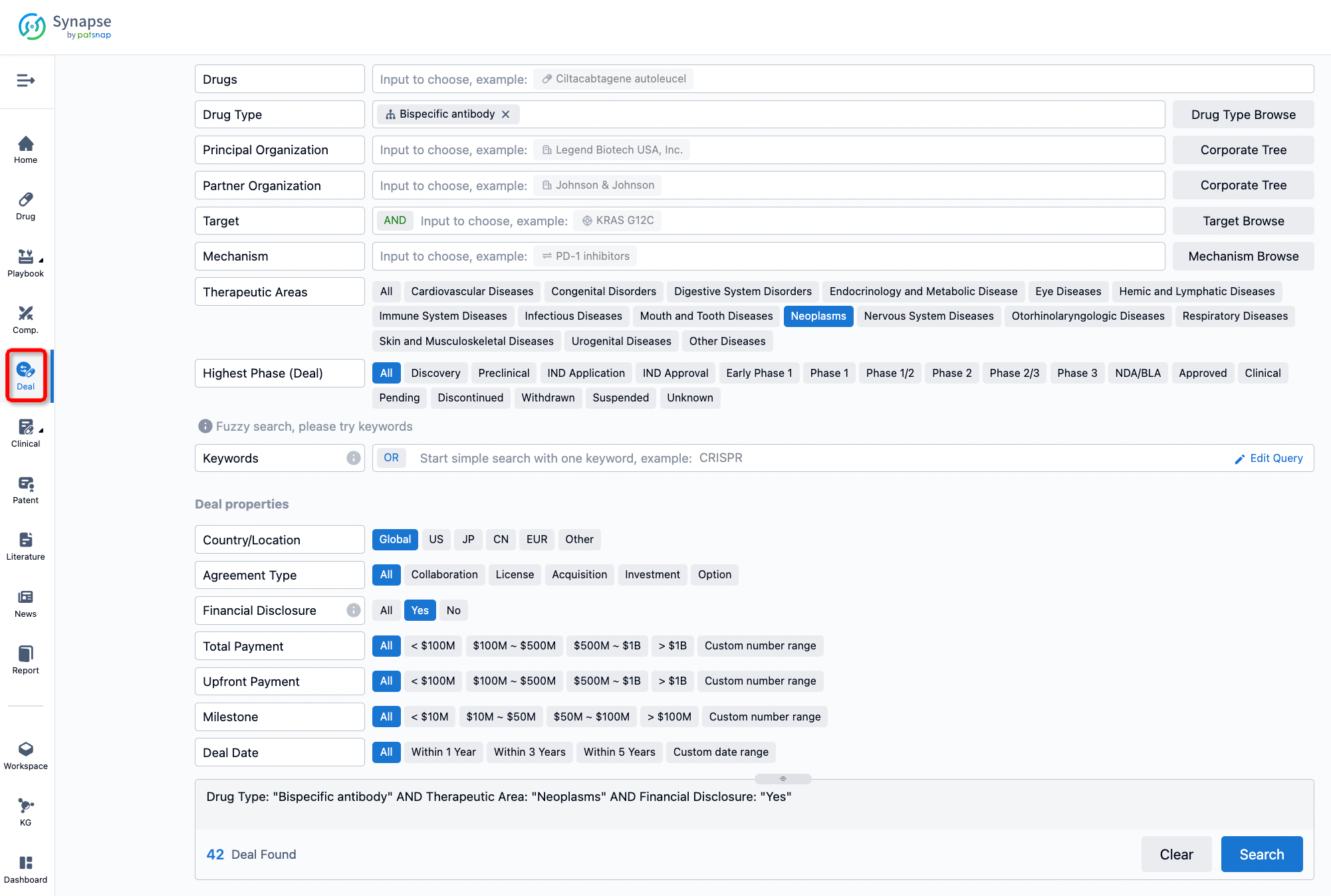
Furthermore, you can obtain the original link to the transaction coverage by clicking on the "Deal Name."
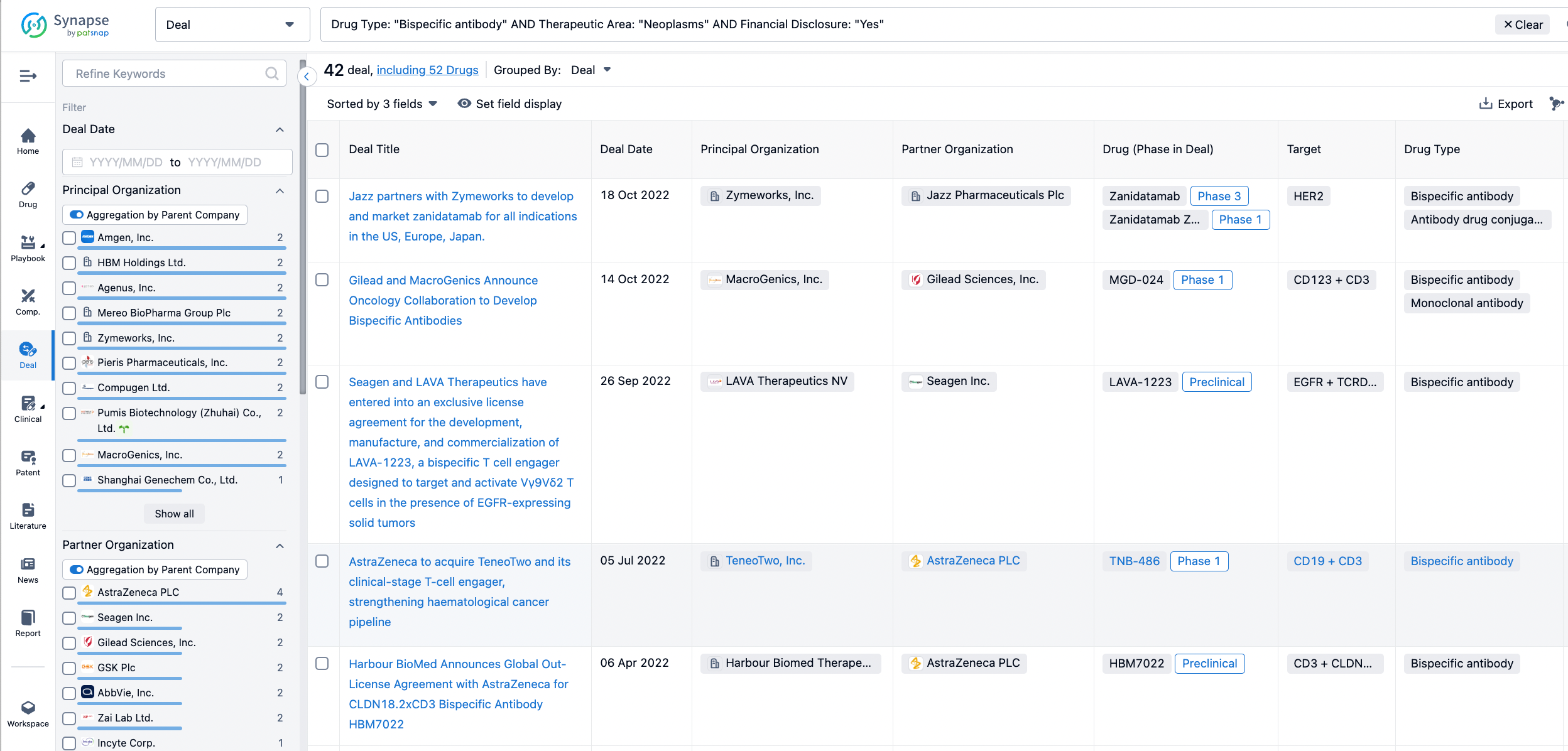
In the analysis view, you can see the most active assignors, assignees, popular targets, and other dimensions of analysis, as well as the distribution of research and development statuses at the time of the transaction, to help you better understand the search results.
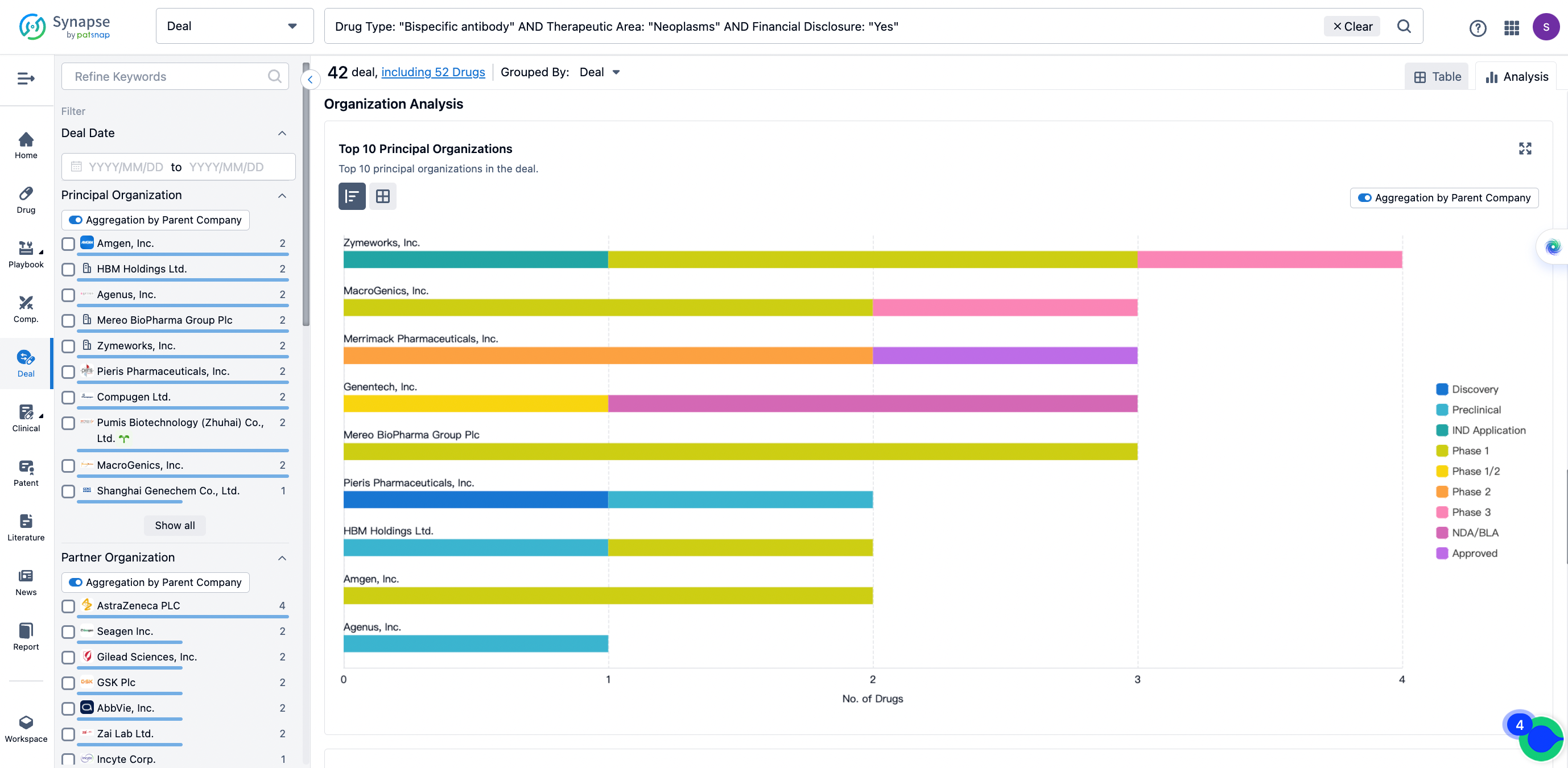
The Synapse database also supports the ability to view current transactions from the dimension of "drugs" (by selecting "drugs" from the "Adjust Dimension" dropdown menu above). Targeting transactions involving renowned pharmaceutical companies that are of interest to the industry, such as Merck, Roche, etc., Synapse has identified a group of "leading companies" through drugs that have achieved global sales exceeding 1 billion US dollars in 2022. Transactions involving drugs from these leading companies can be filtered by clicking on the "Leading Company" tag on the left-hand side.
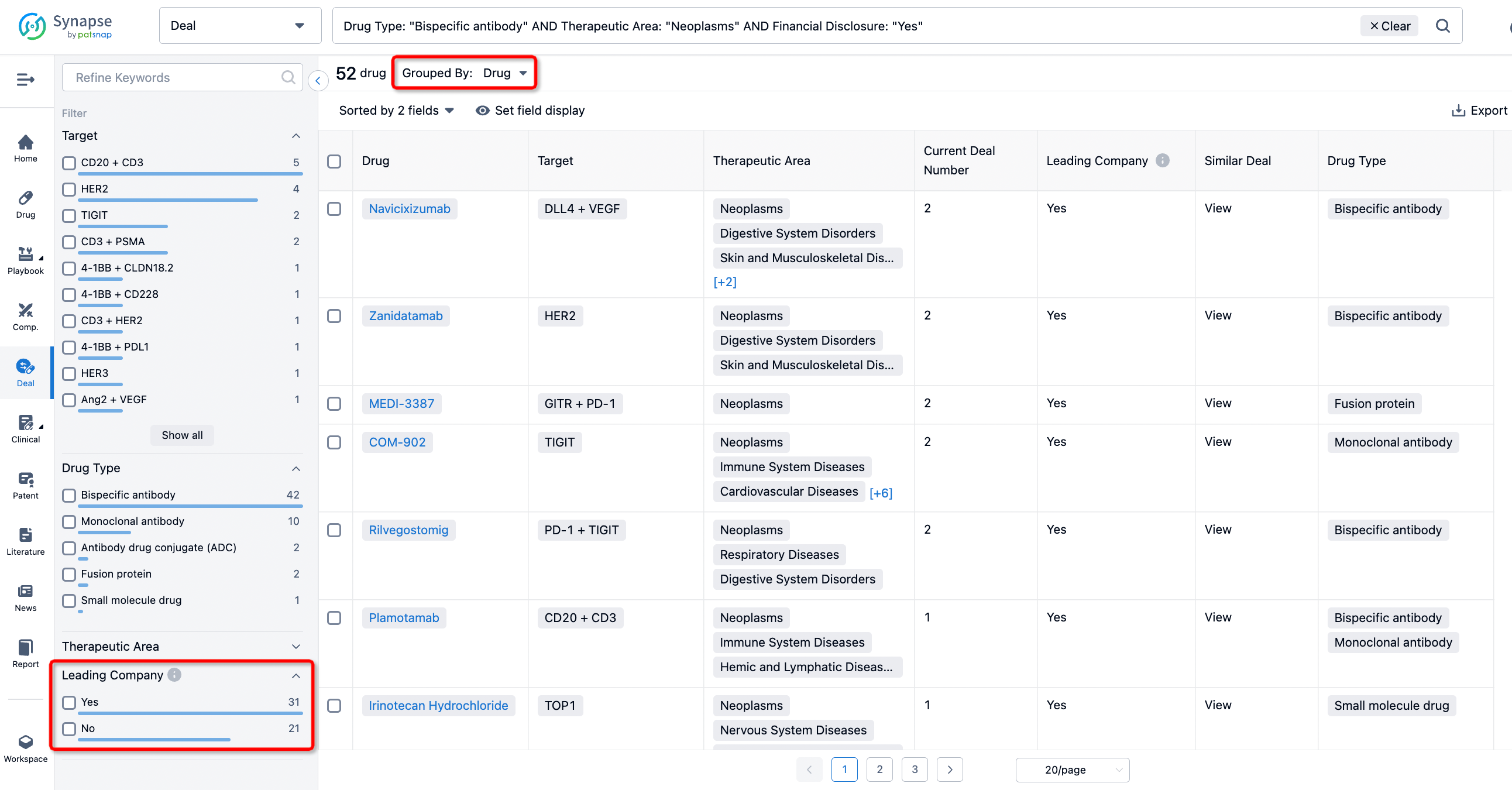
In addition to the drug transaction module, you can also view related transaction history on the drug detail page and the institution detail page.
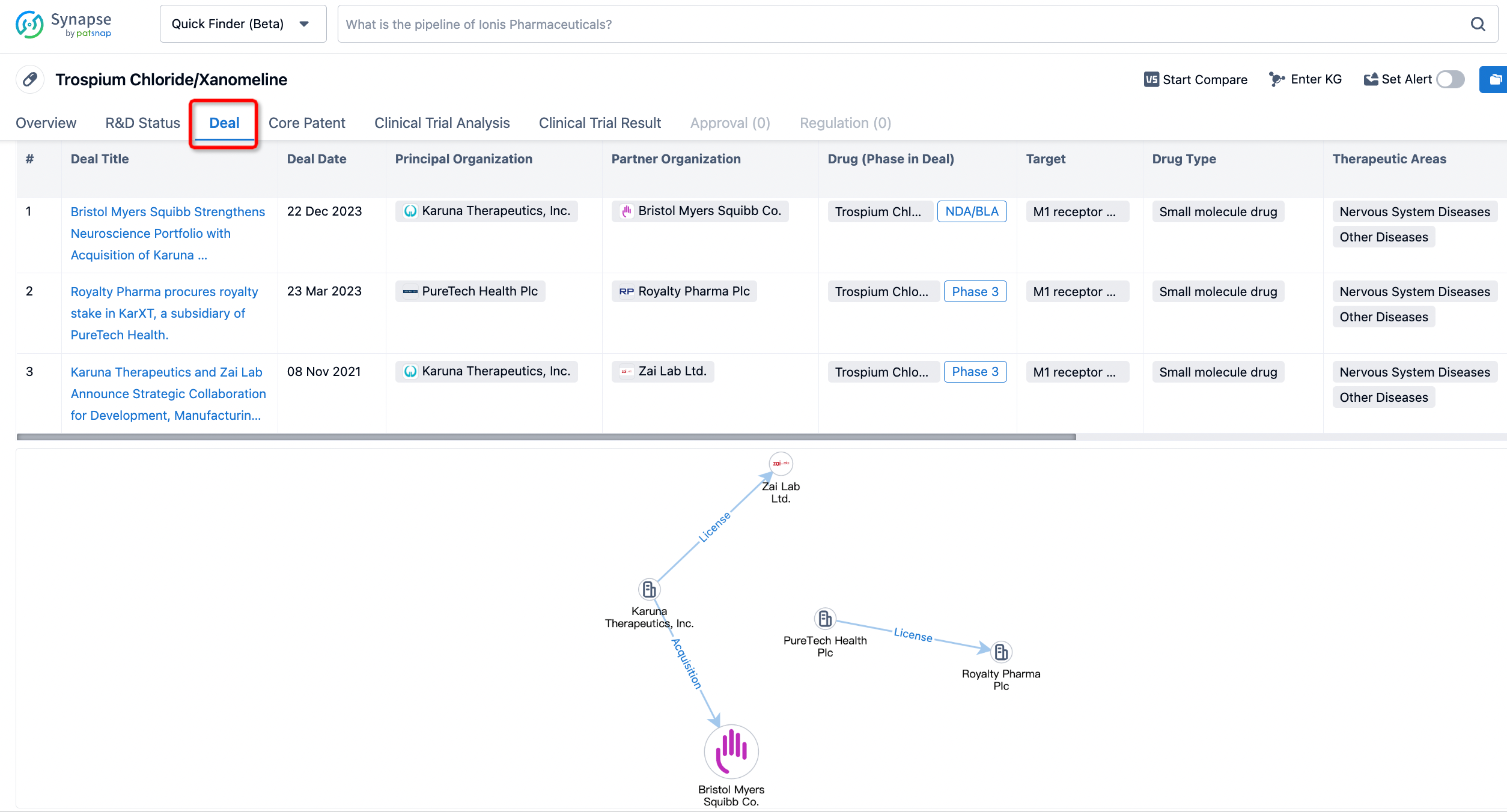
Click on the image below to embark on a brand new journey of drug discovery!