Exploring Zilurgisertib's R&D successes and its clinical results at the 2023 ASH
On 11 Dec 2023, the latest clinical results of the activin receptor-like kinase-2 inhibitor zilurgisertib (INCB000928, LIMBER-104) as monotherapy or with ruxolitinib in patients with anemia due to myelofibrosis will be reported in 2023 ASH.
Zilurgisertib's R&D Progress
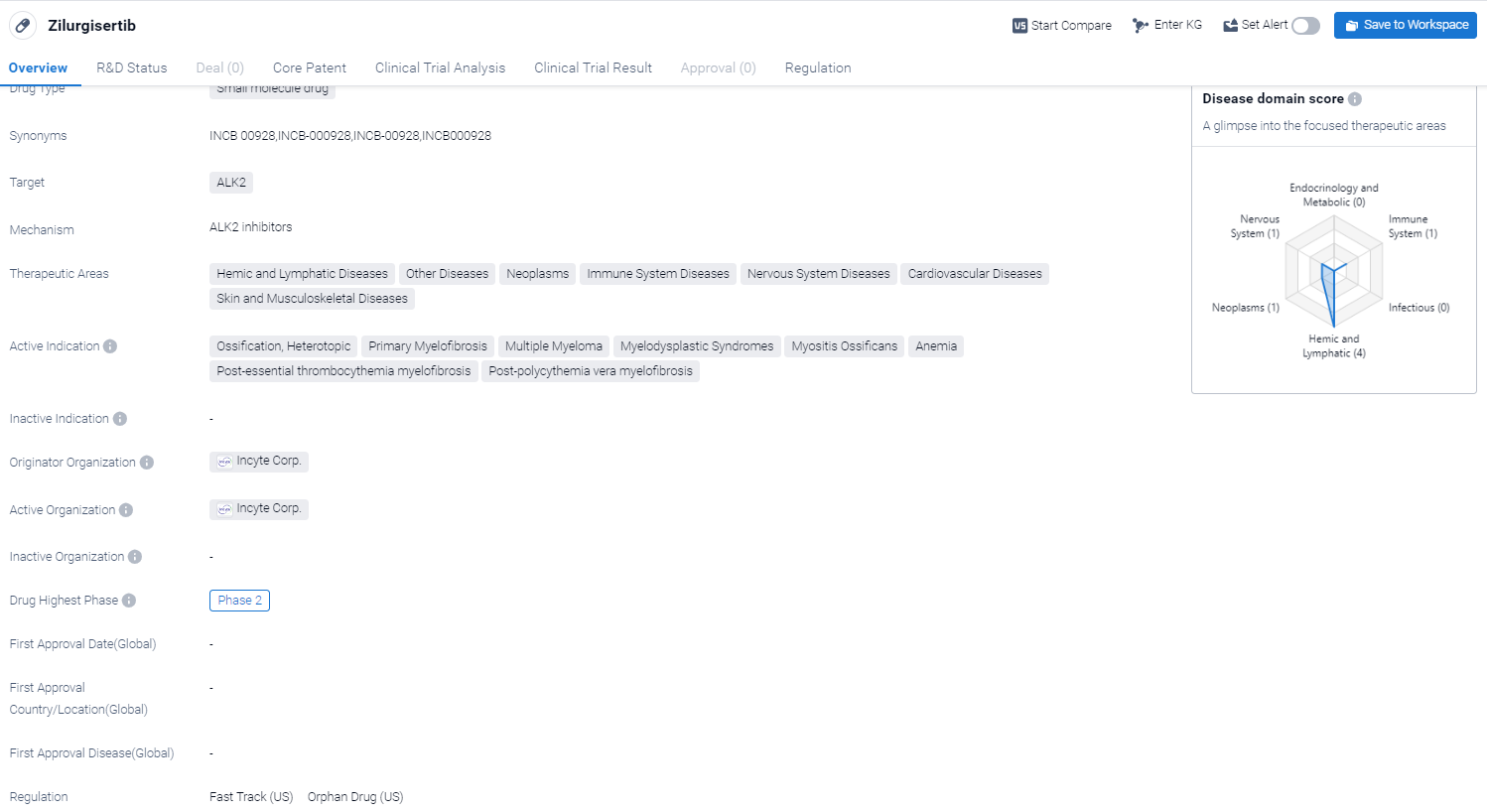
Zilurgisertib is a small molecule drug that targets ALK2, a protein involved in various diseases. It has shown potential therapeutic benefits in a wide range of therapeutic areas, including hemic and lymphatic diseases, neoplasms, immune system diseases, nervous system diseases, cardiovascular diseases, and skin and musculoskeletal diseases.
According to the Patsnap Synapse, currently, Zilurgisertib is in Phase 2 of clinical development globally. And the clinical trial areas for Zilurgisertib are primarily in the United States, China and United Kingdom. The key indication is Myositis Ossificans. 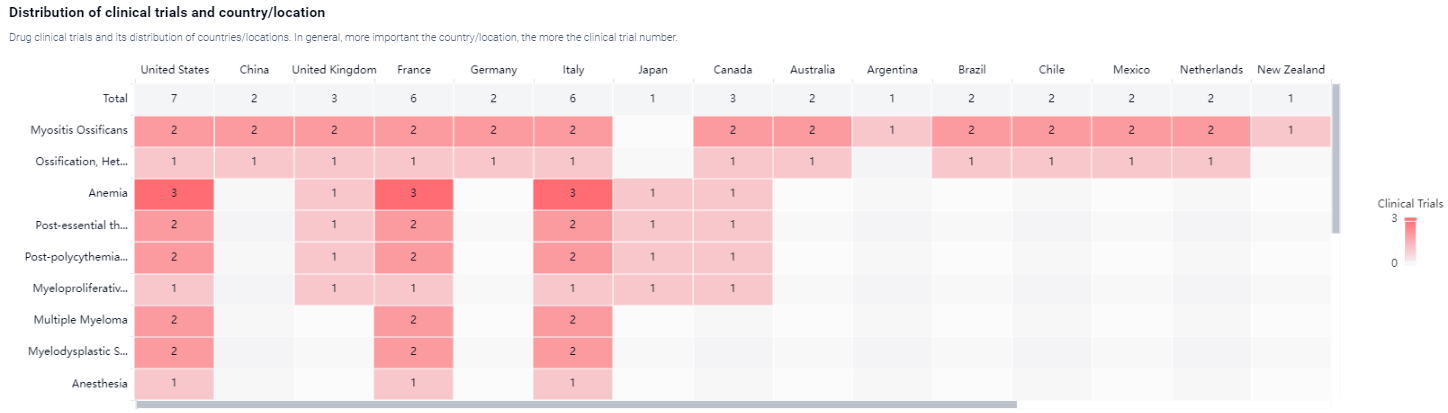
Detailed Clinical Result of Zilurgisertib
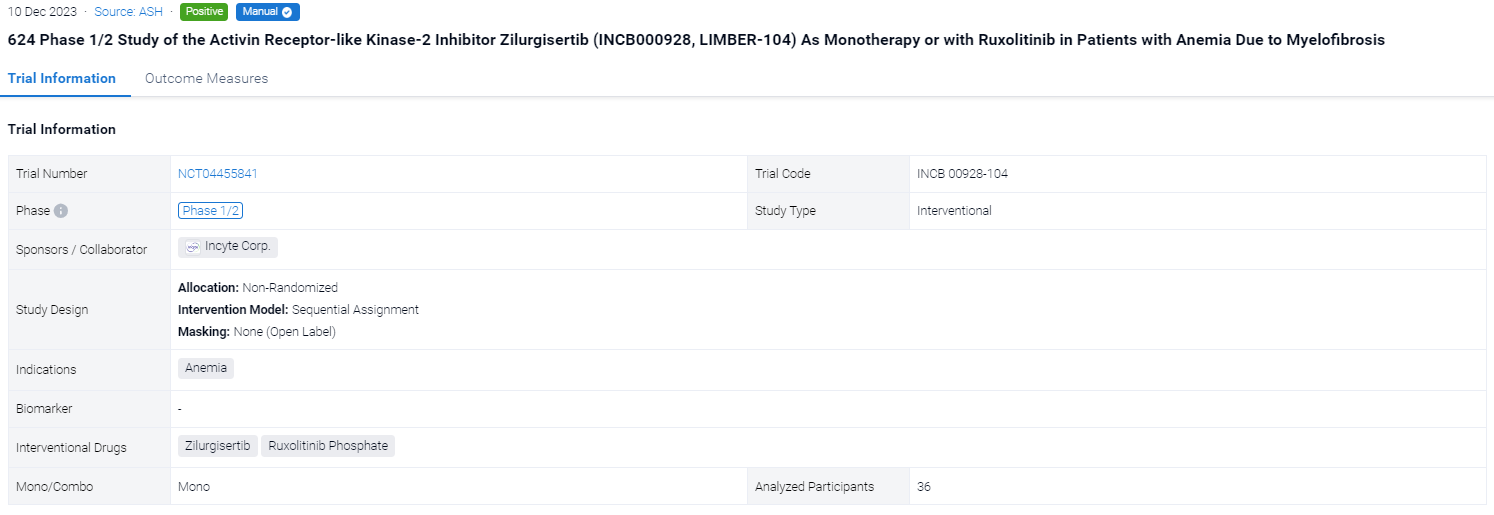
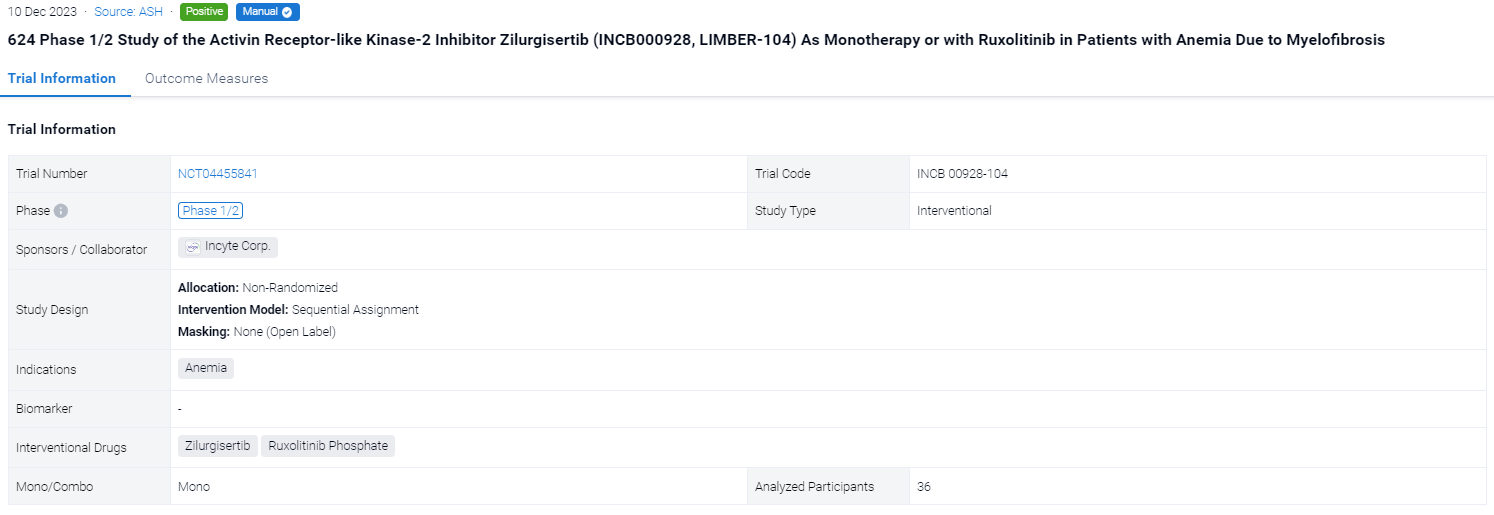
This ongoing open-label, multicenter, phase 1/2 dose-escalation/expansion INCB 00928-104 study (NCT04455841) is evaluating the safety, efficacy, pharmacokinetics (PK), and pharmacodynamics (PD) of zilurgisertib—a potent and selective oral ALK2 inhibitor—in patients (pts) with anemia due to MF.
In this study, eligible pts are ≥18 years old with primary/secondary MF of intermediate (Int)-1 (TGB only) or Int-2/high-risk (TGA and TGB) per the Dynamic International Prognostic Scoring System (DIPSS) and with anemia, including pts who are transfusion dependent (≥4 units of red blood cell transfusions during the 28 days or 8 weeks before Cycle [C] 1 Day [D] 1 for hemoglobin [Hb] <8.5 g/dL in the absence of bleeding or treatment-induced anemia) or are nontransfusion dependent with symptomatic anemia (Hb <10 g/dL during screening on 3 separate occasions ≥7 days apart and not meeting criteria for transfusion dependence). The zilurgisertib starting dose in TGA was 50 mg once daily (qd), with dose increases of ≤2-fold performed until a grade ≥2 toxicity with reasonable probability of being related to the treatment group was observed; subsequent dose increases were limited to ≤50% until the maximum tolerated dose (MTD) was reached or the recommended doses(s) for expansion were identified. In TGB, pts were on a stable regimen of RUX for ≥12 weeks before initiating zilurgisertib at 100 mg qd. The primary endpoints are safety and tolerability, including determination of dose-limiting toxicities (DLTs) and MTD. Secondary endpoints include efficacy (per anemia response parameters), PK, and PD (hepcidin measurement).
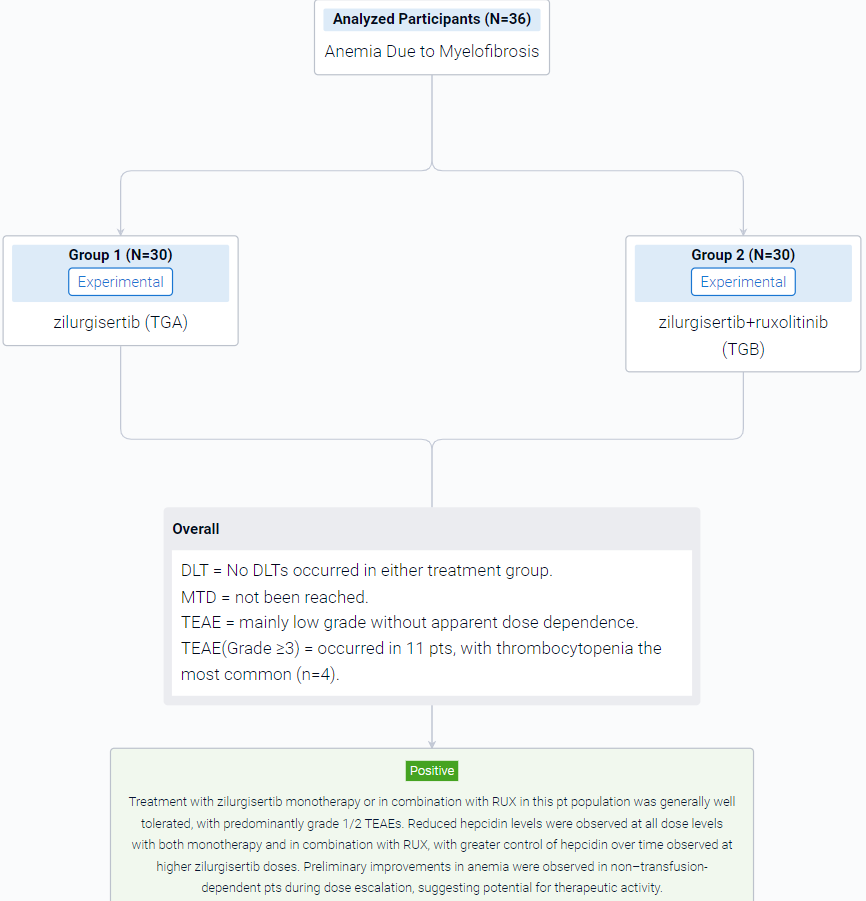
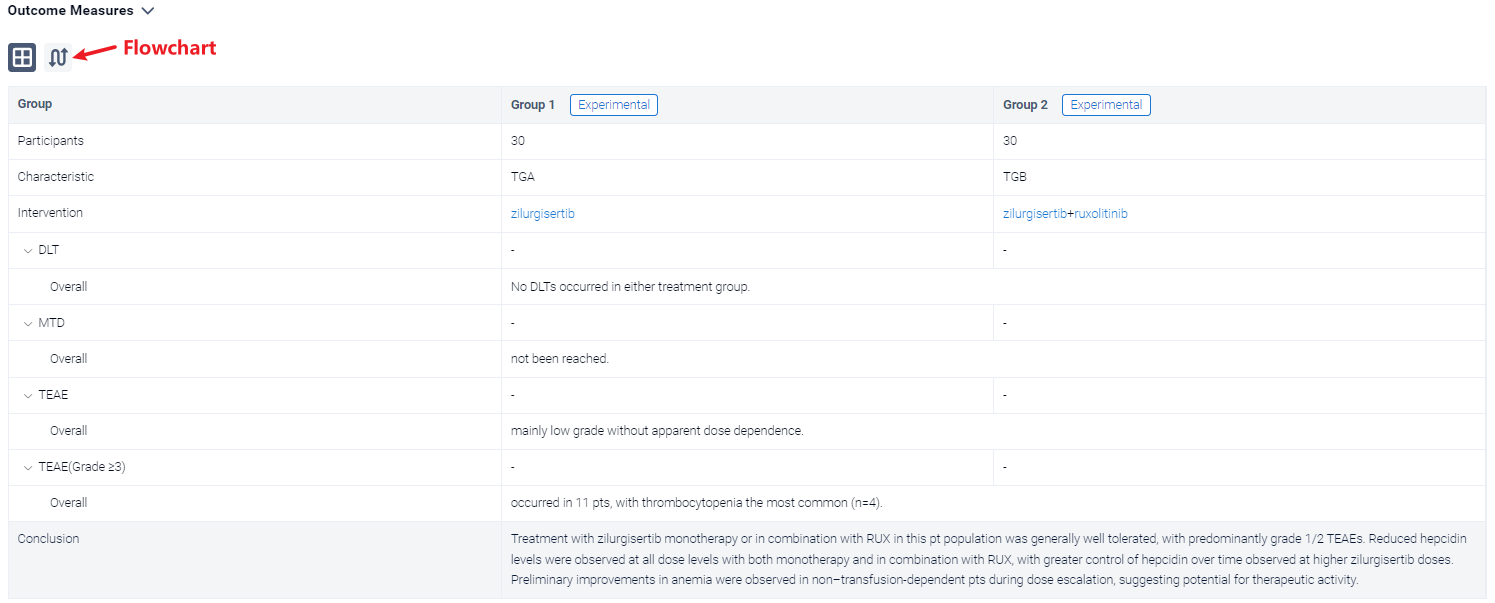
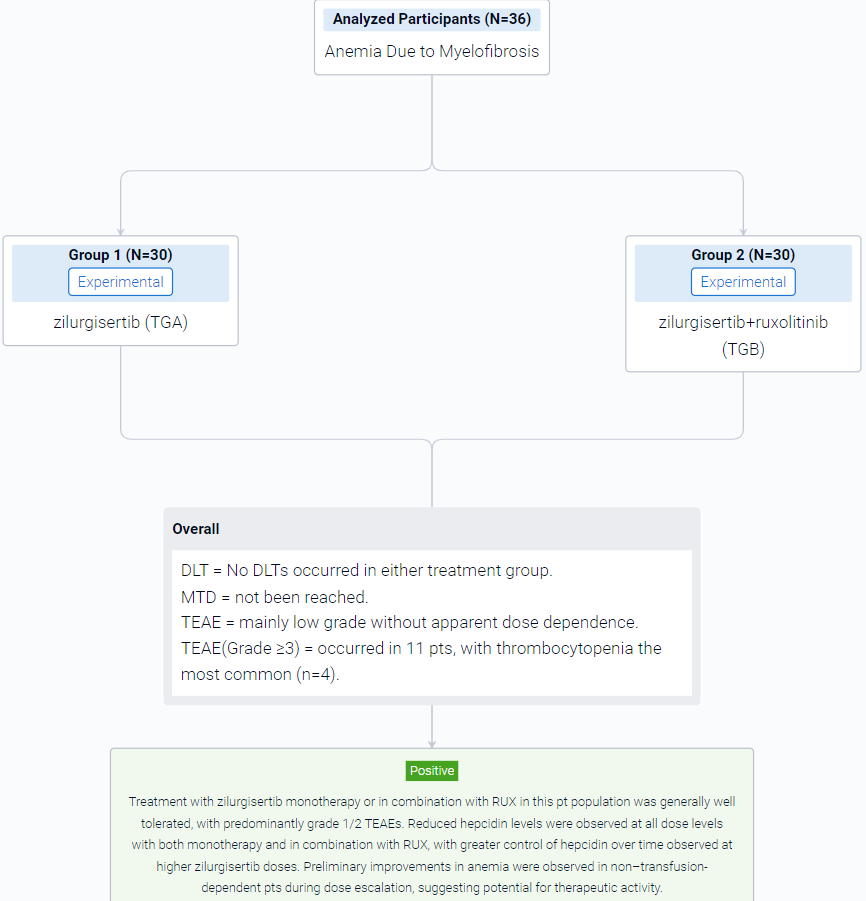
The result showed that a total of 36 pts were enrolled at the time of analysis (data cutoff, February 15, 2023), with 20 in TGA and 16 in TGB. Median (range) age was 73 (53–84) years for TGA and 75.5 (54–85) years for TGB; 65% of TGA pts had received prior RUX therapy. Overall, 89% had Int-2 risk DIPSS, and the remainder had high-risk DIPSS (1 in TGA, 3 in TGB). In total, 55% of pts in TGA and 25% in TGB were transfusion dependent at time of enrollment. Median (range) baseline Hb was 7.7 (7–10) g/dL in TGA and 8.0 (5–9) g/dL in TGB. Baseline median (range) hepcidin was high in both cohorts (TGA, 171 [18–535] ng/mL; TGB, 126 [7–421] ng/mL; normal range, 0–50 ng/mL). At the time of analysis, dose escalation was ongoing in both treatment groups. No DLTs occurred in either treatment group, and the MTD had not been reached. Treatment-emergent adverse events (TEAEs) were mainly low grade without apparent dose dependence. Grade ≥3 TEAEs occurred in 11 pts, with thrombocytopenia the most common (n=4). The PK profile for zilurgisertib at steady state was consistent with that observed with previous healthy volunteer studies, with observed time of maximum concentration at 2–4 hours after administration and predicted half-life of approximately 24 hours. Maximum reduction in hepcidin following zilurgisertib dosing in C1D1 was observed at 6–8 hours postdose; the highest doses tested in both TGA and TGB demonstrated more effective maintenance of hepcidin suppression over time compared with lower doses. Among non–transfusion-dependent pts, anemia improvement (Hb increase of ≥1.5 g/dL vs baseline) was observed in both treatment groups (Figure). Among transfusion-dependent pts, anemia response of transfusion independence had not been observed at data cutoff. All pts with anemia improvement have maintained Hb increase in the absence of transfusion and remain on study.
It can be concluded that treatment with zilurgisertib monotherapy or in combination with RUX in this pt population was generally well tolerated, with predominantly grade 1/2 TEAEs. Reduced hepcidin levels were observed at all dose levels with both monotherapy and in combination with RUX, with greater control of hepcidin over time observed at higher zilurgisertib doses. Preliminary improvements in anemia were observed in non–transfusion-dependent pts during dose escalation, suggesting potential for therapeutic activity.
How to Easily View the Clinical Results Using Synapse Database?
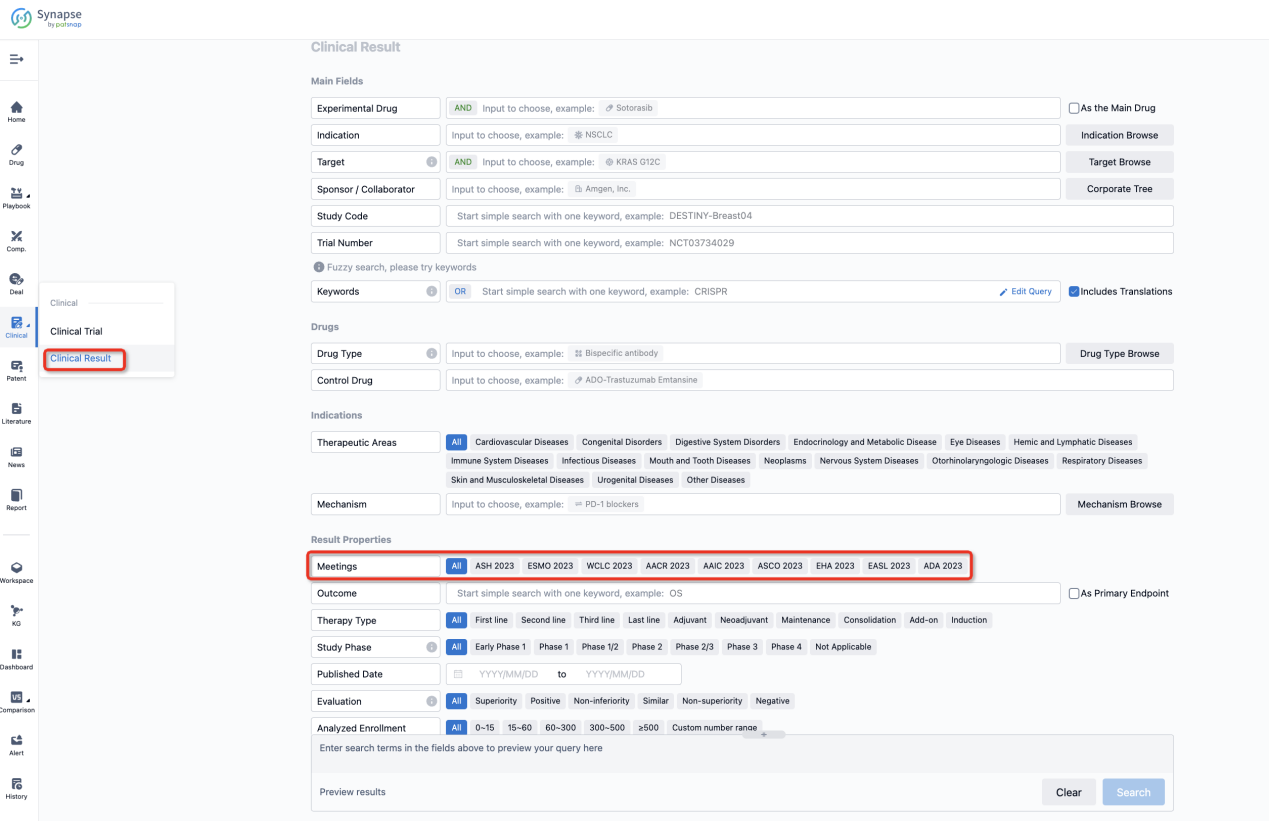
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
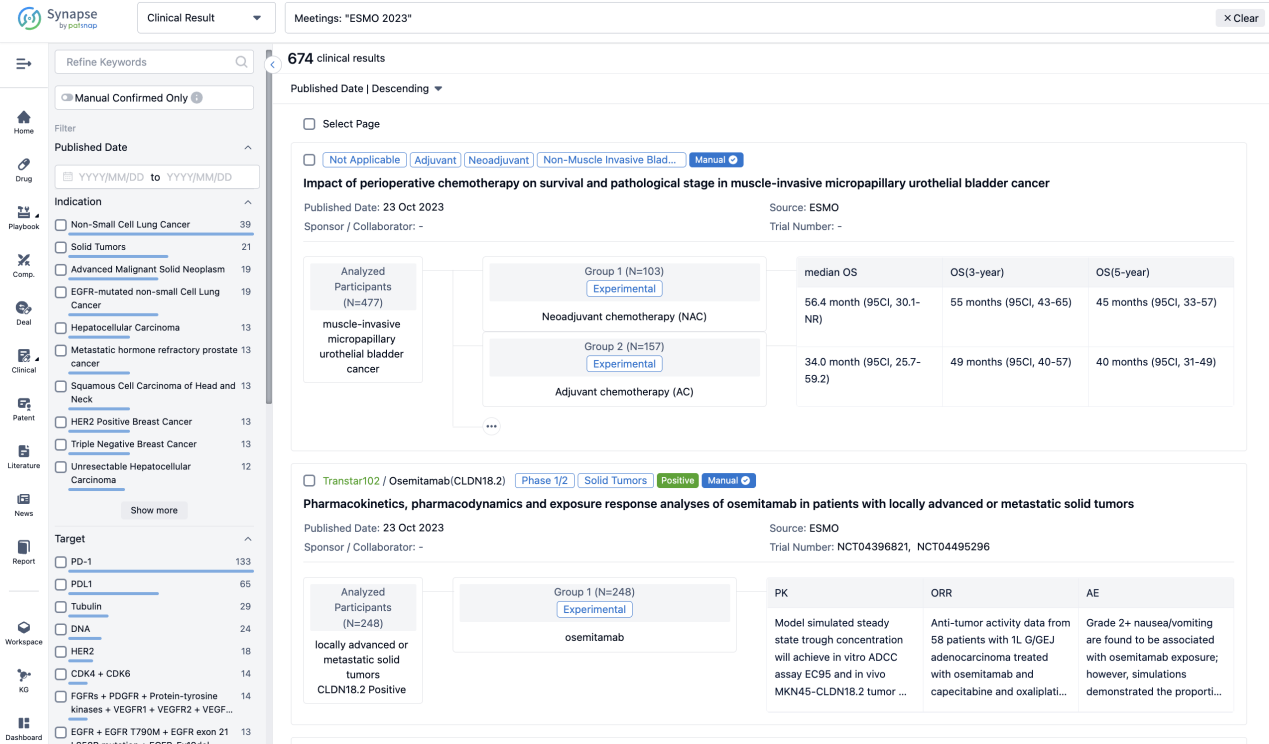
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
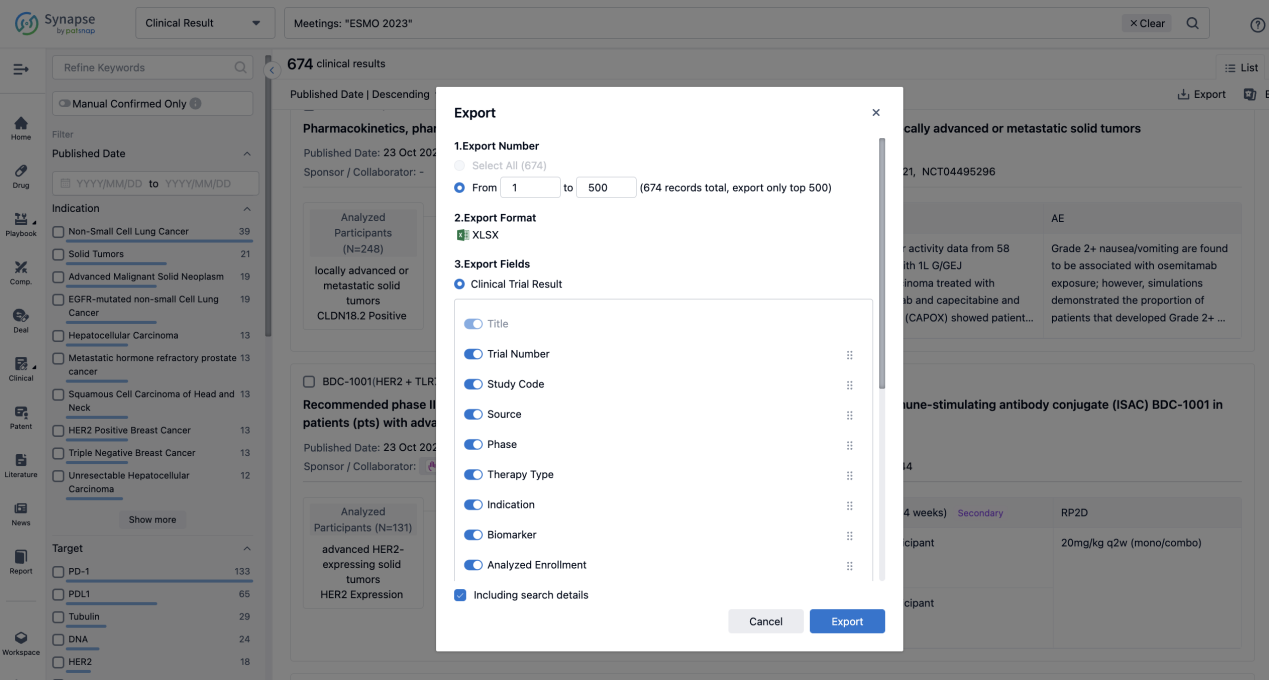
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!