FDA approves Century Therapeutics' CNTY-101 for systemic lupus erythematosus treatment
Century Therapeutics, a pioneering enterprise focused on creating cell treatments from induced pluripotent stem cells (iPSC) for use in immunotherapy for cancer as well as autoimmune and inflammatory conditions, revealed today that the U.S. Food and Drug Administration (FDA) has given the green light for its Phase 1 clinical study. This initial stage of testing will evaluate the efficacy of CNTY-101 on individuals with systemic lupus erythematosus of a moderate to severe classification, targeting those who have not responded adequately to a minimum of two conventional immunosuppressive treatments.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
This marks a key development for CNTY-101, being the inaugural clearance of an Investigational New Drug (IND) submission for its use in the treatment of an autoimmune and inflammatory condition. This progress is underpinned by the insights and clinical experience obtained from the administration of several rounds of CNTY-101 to patients, both with and without lymphodepletion protocols, based on the existing open IND related to its use in B-cell malignancies that are relapsed or refractory.
In the landscape of Systemic Lupus Erythematosus (SLE), recent therapeutic approaches have yet to significantly reduce disease-related complications, and the attainment of remission is infrequent. Common concerns include adverse reactions to treatments and the frequent recurrence of disease symptoms. B-cell-targeting autologous CAR T cell treatments have emerged as a promising strategy offering sustained periods of remission through the mechanism of "immune system resetting."
Challenges similar to those observed in the use of auto-CAR-T in cancer treatments, such as issues related to the accessibility of the treatment, adverse effects, and prospective long-term risks, might limit the broad implementation of these therapies in non-cancerous conditions. Century Therapeutics is advancing a different strategy via CNTY-101, which is an allogeneic, readily available NK cell therapy product derived from induced pluripotent stem cells (iPSCs). It comprises six genetic modifications at specified genomic positions, which have been tailored through the precision of homology-directed repair facilitated by CRISPR technology.
Century Therapeutics' CEO, Brent Pfeiffenberger, Pharm.D., announced, "Achieving this milestone underscores Century's progressive development. We are confident in CNTY-101's distinct characteristics, which feature extensive precision genomic modifications, including our proprietary Allo-Evasion™ technology. This positions the therapy as a non-donor-dependent allogeneic option, which can potentially enhance the health outcomes for those SLE patients who have found limited success with current treatment avenues."
Dr. Pfeiffenberger further remarked, "Expanding the CNTY-101 initiative to include SLE management is a notable success for our company. Moreover, this advancement reflects the tireless commitment of our team to fulfill our guiding principle of delivering innovative and potentially life-altering cell therapies to individuals living with cancer, as well as those facing autoimmune and inflammatory diseases."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
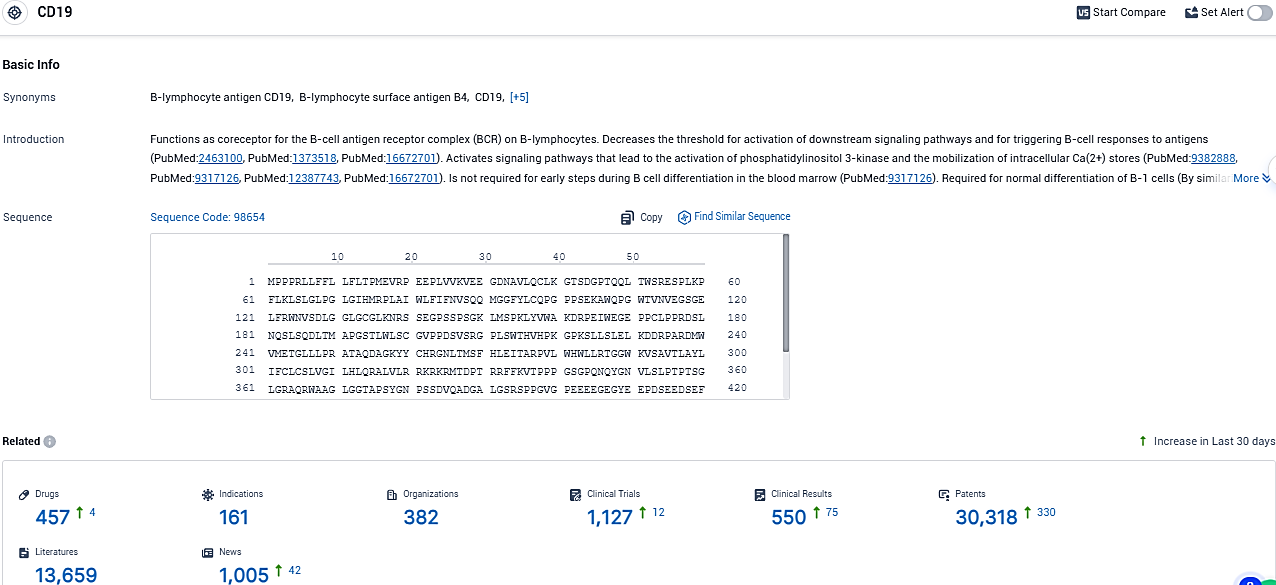
According to the data provided by the Synapse Database, As of December 15, 2023, there are 457 investigational drugs for the CD19 target, including 161 indications, 382 R&D institutions involved, with related clinical trials reaching 1127, and as many as 30318 patents.
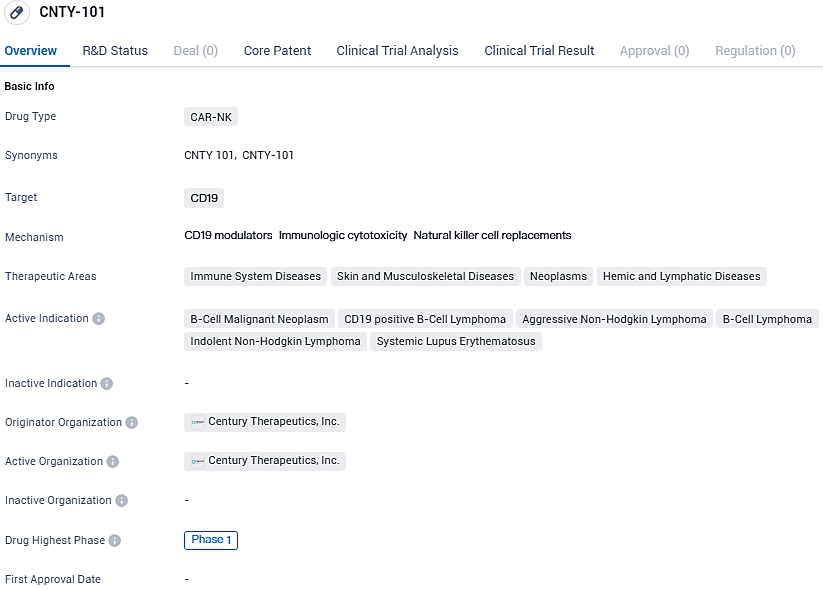
CNTY-101 is an investigational off-the-shelf immunotherapy product candidate that utilizes iPSC-derived natural killer cells with a CD19-directed chimeric antigen receptor. It is being developed for the treatment of various diseases related to the immune system, skin and musculoskeletal system, neoplasms, and hemic and lymphatic diseases.