FDA Approves TransCode's Phase 1/2 Trial of TTX-MC138 for Advanced Solid Tumors
TransCode Therapeutics, Inc., a specialist in RNA oncology, has revealed that the U.S. Food and Drug Administration has finished reviewing their Investigational New Drug application and has approved the commencement of a Phase 1/2 study. This trial is a multicenter, open-label investigation that includes both dose-escalation and dose-expansion phases, focusing on their principal therapeutic agent, TTX-MC138, for treating patients with advanced solid tumors.
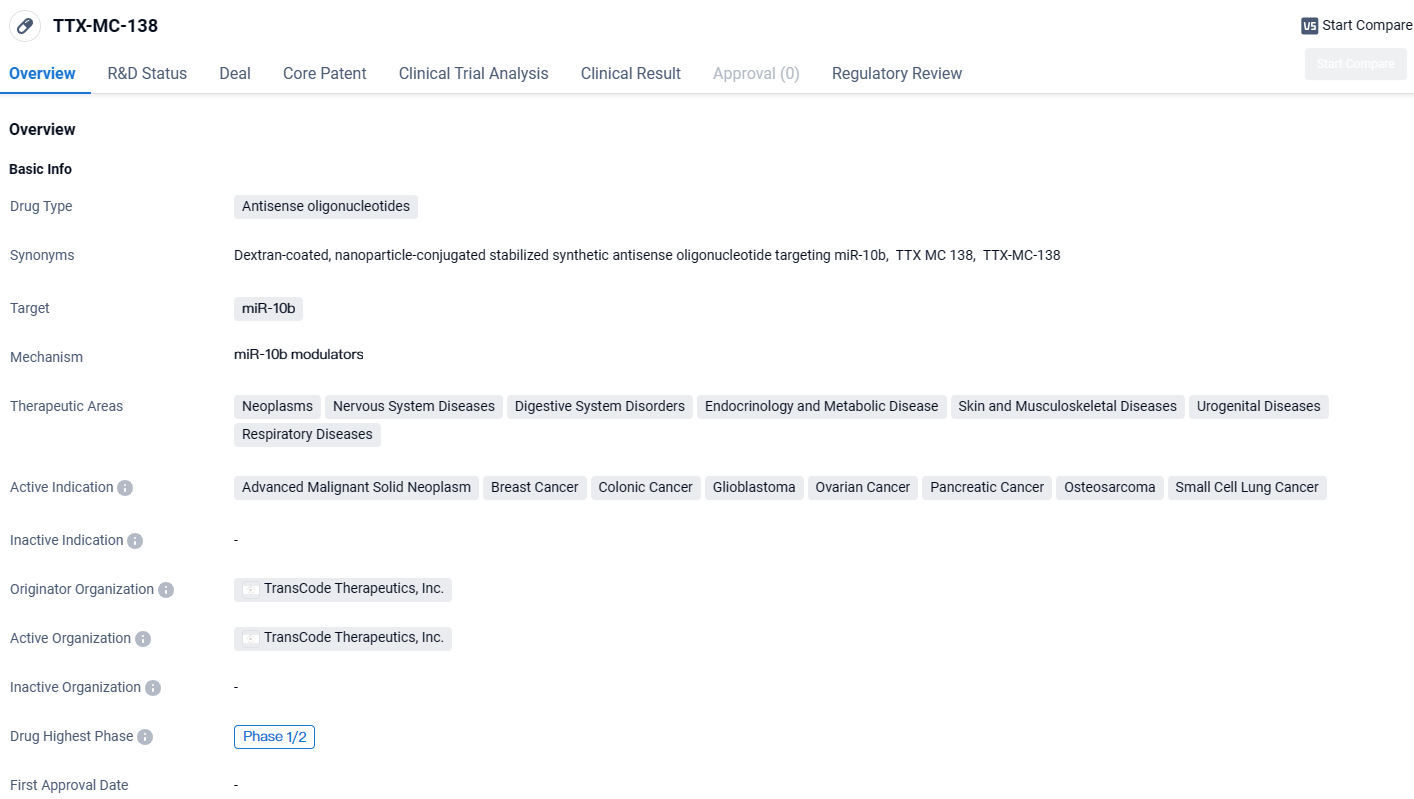
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
“We are excited to announce the FDA’s authorization to move TTX-MC138 into clinic trials," stated Sue Duggan, Senior Vice President of Operations at TransCode. "Gaining FDA approval for our IND application is a pivotal moment for our institution," she added.
Sue Duggan continued, “With the IND activated, we can proceed with further development of TTX-MC138 within a clinical environment. The upcoming Phase 1/2 clinical trial aims to gather vital data that will aid in assessing TTX-MC138’s safety and could demonstrate preliminary effectiveness in treating patients with metastatic conditions.”
The structured Phase 1/2 clinical trial will start with a dose-escalation stage, followed by a dose-expansion stage. The trial first targets varying metastatic solid tumors during the dose-escalation part, focusing on assessing the safety and tolerability of increasing doses of TTX-MC138.
Following favorable outcomes in the initial phase, the dose-expansion stage will concentrate on specific cancer types. It aims to further investigate the safety, tolerability, and potential anti-tumor capabilities of TTX-MC138.
TransCode believes TTX-MC138 holds promise in improving treatment outcomes across various cancers such as breast, pancreatic, ovarian, colon, and glioblastomas, among others. In several cancer animal models, TTX-MC138 treatment significantly decreased metastatic progression and increased survival rates, compared to controls. The successful progression of TTX-MC138 through clinical trials could offer a groundbreaking treatment option for patients battling metastatic cancer.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
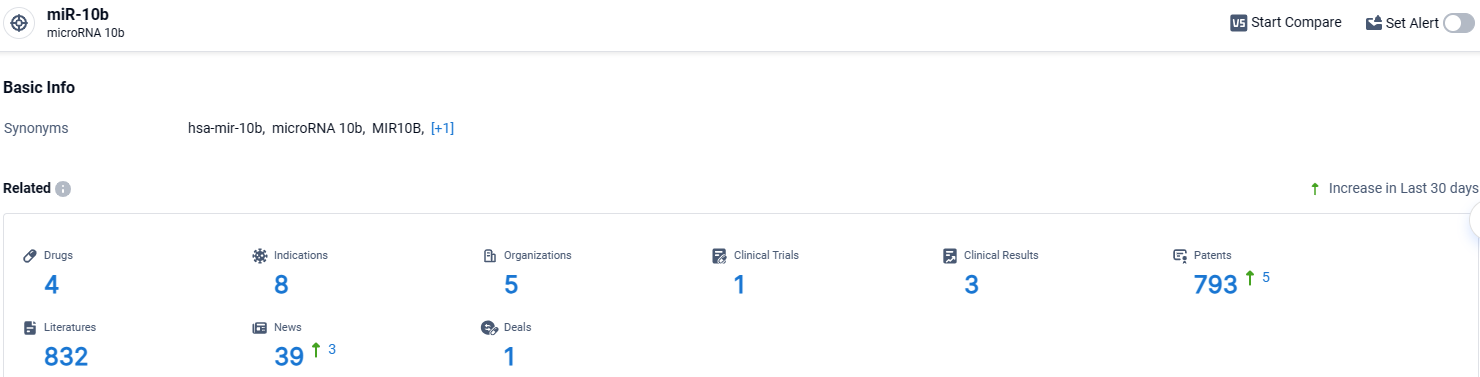 According to the data provided by the Synapse Database, As of April 17, 2024, there are 4 investigational drugs for the miR-10b target, including 8 indications, 5 R&D institutions involved, with related clinical trials reaching 1, and as many as 793 patents.
According to the data provided by the Synapse Database, As of April 17, 2024, there are 4 investigational drugs for the miR-10b target, including 8 indications, 5 R&D institutions involved, with related clinical trials reaching 1, and as many as 793 patents.
TTX-MC-138 targets miR-10b and has potential applications in the treatment of various cancers and diseases affecting different body systems. The drug is currently in Phase 1/2 of development and has been designated as an orphan drug, indicating its potential to address unmet medical needs in rare diseases. Further research and clinical trials are needed to determine the drug's efficacy and safety profile.