FDA Grants Accelerated Approval for KRAZATI® and Cetuximab Combo in Advanced KRAS G12C Mutated CRC
Bristol Myers Squibb has revealed that the U.S. Food and Drug Administration (FDA) has given expedited approval for KRAZATI (adagrasib) to be used in conjunction with cetuximab as a targeted therapy for adults diagnosed with KRAS G12C-mutated locally advanced or metastatic colorectal cancer. This approval applies to patients identified using an FDA-approved test who have previously undergone treatment with chemotherapy regimens that include fluoropyrimidine, oxaliplatin, and irinotecan.
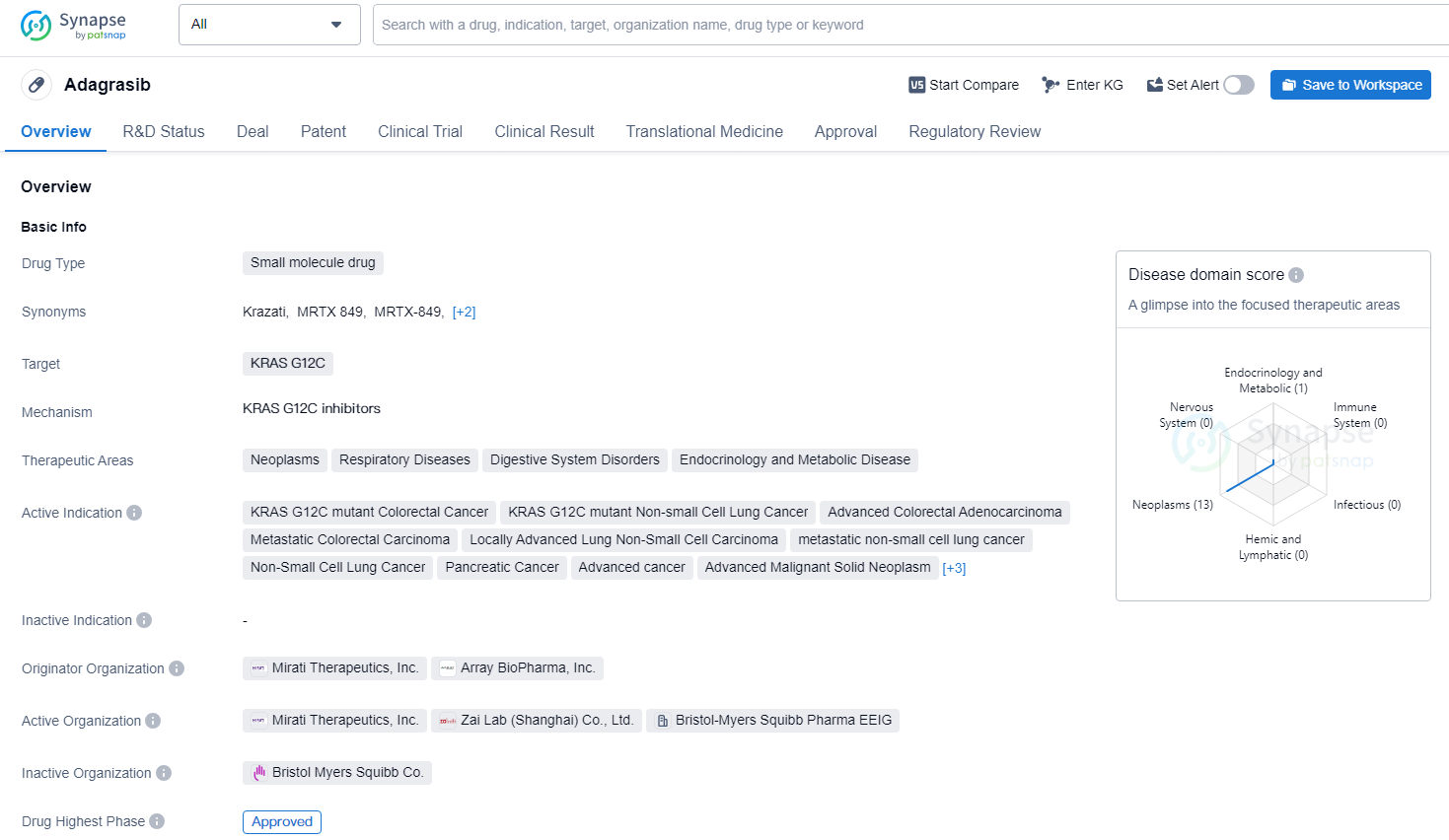
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
This indication has been granted accelerated approval based on the findings related to objective response rate and the duration of response. Future approval for this indication might depend on confirming and outlining clinical benefits through a confirmatory study.
"CRC with a KRASG12C mutation presents in about 3-4% of CRC patients and has been historically difficult to tackle," noted Dr. Rona Yaeger, a Gastrointestinal Oncologist and Early Drug Development Specialist at Memorial Sloan Kettering Cancer Center. "The FDA's approval of KRAZATI in combination with cetuximab offers a new treatment avenue for these patients whose tumors have shown poor response to previous therapies."
The approval stems from findings within cohorts of the Phase 1/2 KRYSTAL-1 open-label study, which assessed KRAZATI paired with cetuximab in 94 heavily pretreated CRC patients with a KRASG12C mutation. The study reached its primary endpoint, showing a confirmed ORR of 34% for KRAZATI with cetuximab, all of which were partial responses. The median DOR, a secondary endpoint, stood at 5.8 months. Current standard-of-care options for late-line treatment show limited response rates post-progression on chemotherapy ± VEGF/VEGFR inhibitors.
KRAZATI comes with the following Warnings & Precautions: gastrointestinal adverse reactions like diarrhea, nausea, and vomiting, QTc interval prolongation, hepatotoxicity, and interstitial lung disease/pneumonitis. For more details, please review the Important Safety Information below.
In 2022, the FDA awarded breakthrough therapy designation to KRAZATI in combination with cetuximab for patients with KRASG12C-mutated advanced CRC whose cancer has advanced following prior chemotherapy and an anti-VEGF therapy.
KRAZATI is an irreversible inhibitor of KRASG12C, featuring a long half-life, dose-dependent pharmacokinetics, and central nervous system penetration. When used in combination with cetuximab, it may bolster the inhibition of KRAS-dependent signaling or counteract adaptive feedback mechanisms.
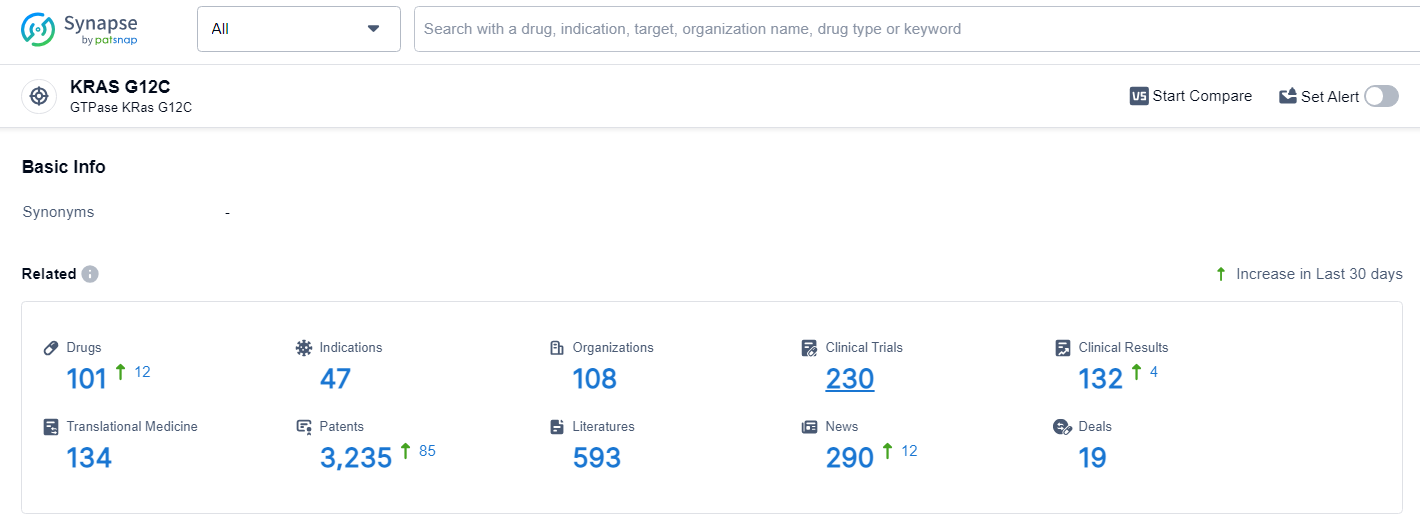
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 27, 2024, there are 101 investigational drugs for the KRAS G12C target, including 47 indications, 108 R&D institutions involved, with related clinical trials reaching 230, and as many as 3235 patents.
Adagrasib represents a promising development in the field of biomedicine, particularly in the treatment of various cancers and related diseases associated with the KRAS G12C mutation. The drug's broad therapeutic areas and the regulatory support it has received suggest its potential to make a significant impact on patient care and outcomes.