FDA Greenlights Phase II/III Trial of KPG-121 Coupled with Abiraterone for Initial Therapy in mCRPC
Kangpu Biopharmaceuticals recently revealed that the U.S. Food and Drug Administration has granted approval for a Phase II/III clinical trial investigating KPG-121 in combination with Abiraterone as a first-line therapy for metastatic castration-resistant prostate cancer.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
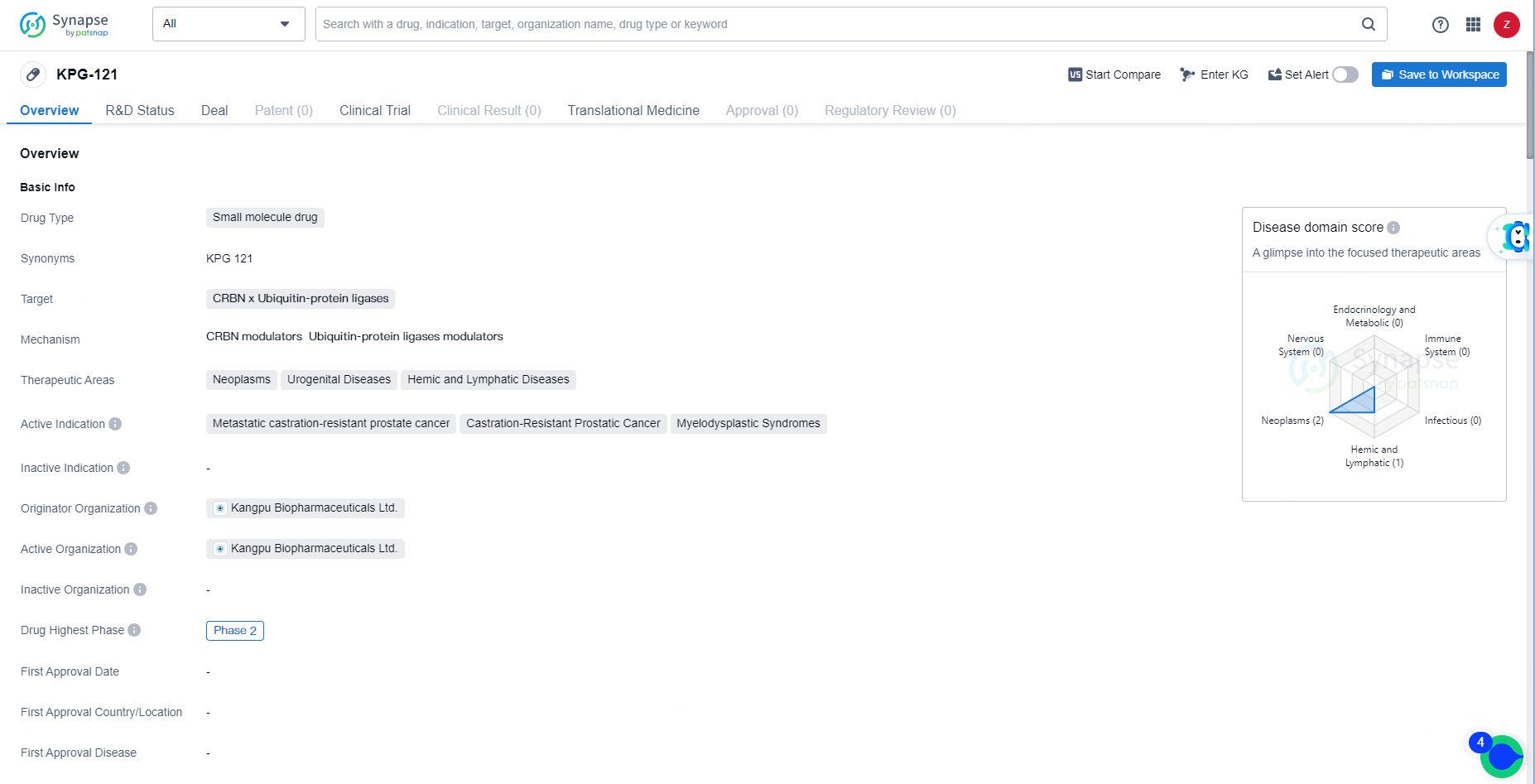
Discovered by Kangpu Biopharmaceuticals, KPG-121 functions as a modulator of the Cereblon (CRBN) E3 ubiquitin ligase complex CRL4CRBN. It is designed to facilitate the rapid ubiquitination and degradation of casein kinase 1α (CK1α) and transcription factors Aiolos (IKZF3) and Ikaros (IKZF1).
KPG-121 exhibits properties that inhibit cell proliferation and new blood vessel formation, while also boosting immunomodulatory effects. When combined with androgen-receptor antagonists such as enzalutamide, abiraterone acetate, apalutamide, or darolutamide, KPG-121 markedly enhances anti-tumor effectiveness in xenograft models compared to using androgen-receptor antagonists alone.
A Phase I clinical trial conducted in the United States assessed the safety, pharmacokinetics, and therapeutic efficacy of KPG-121 in combination with enzalutamide, abiraterone, or apalutamide for treating patients with both metastatic and non-metastatic castration-resistant prostate cancer. The study found that KPG-121 was generally well tolerated, had a favorable pharmacokinetic profile, and showed promising therapeutic efficacy.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
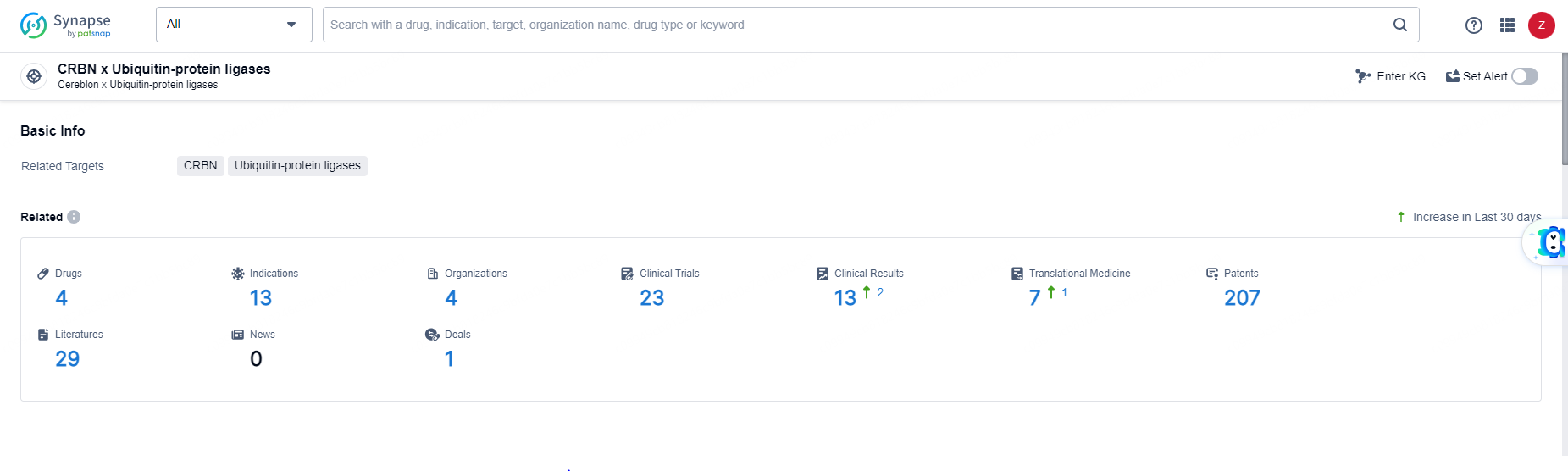
According to the data provided by the Synapse Database, As of June 11, 2024, there are 4 investigational drugs for the CRBN and Ubiquitin-protein ligases target, including 13 indications, 4 R&D institutions involved, with related clinical trials reaching 23, and as many as 207 patents.
KPG-121 is a small molecule drug with a focus on addressing neoplasms, urogenital diseases, and hemic and lymphatic diseases. Its active indications include metastatic castration-resistant prostate cancer, castration-resistant prostatic cancer, and myelodysplastic syndromes. With its progression to Phase 2 globally and Phase 1 in China, KPG-121 represents a promising candidate for the treatment of these challenging medical conditions.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!