First participant receives Cu-67 SAR-Bombesin treatment in theranostic prostate cancer study
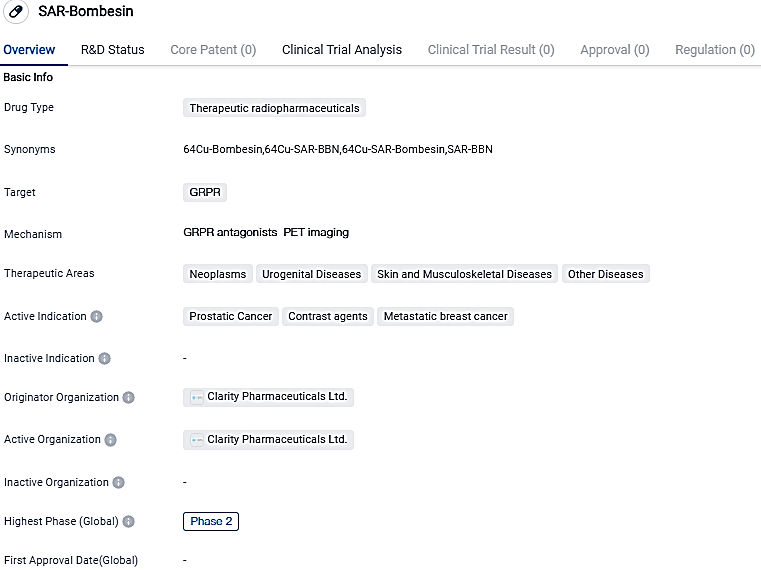
Clarity Pharmaceuticals, a developing company that specializes in clinical-stage radiopharmaceuticals with a core focus on revolutionizing cancer treatments for adults and children, proudly announces the initial patient dosing in their 64Cu/67Cu SAR-Bombesin Phase I/II theranostic trial for patients with metastatic castrate resistant prostate cancer.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
During the application of 6GBq of 67Cu SAR-Bombesin, no issues were observed, and the participant still receives continuous follow-up checks to evaluate safety and effectiveness according to the guidelines.
COMBAT, short for "Copper-67 SAR-Bombesin in advanced castrate-resistant prostate cancer," is a clinical trial that intends to gradually increase dosage and expand the participant cohort to possibly 38 individuals. 64Cu SAR-Bombesin is utilized to locate gastrin-releasing peptide receptor (GRPr)-expressing lesions and select suitable candidates for subsequent 67Cu SAR-Bombesin therapy.
The trial's goal is to verify the security and effectiveness of 67Cu SAR-Bombesin in participants having GRPr expressing mCRPC, who are ineligible for therapy with 177Lu PSMA-617 treatment. These individuals, unlikely to gain from prostate-specific membrane antigen (PSMA)-targeted agents, signify a significant unfilled gap in both imaging and therapy. The trial intends to delve into the administration of up to 14GBq of 67Cu SAR-Bombesin in a maximum of four cycles.
Clarity's Head, Dr Alan Taylor, said, "Through diagnostic trials, SAR-Bombesin has contributed to the better management of prostate cancer in patients with low or no PSMA expressing lesions, and we aim to corroborate its safety and effectiveness in this theranostic trial. We are eager to advance the COMBAT trial, taking into account the persuasive data derived from our previous preclinical and clinical research. This, combined with the operational and manufacturing advantages of Targeted Copper Theranostics."
The highly targeted pan-cancer radiopharmaceutical SAR-Bombesin is applicable to various types of cancer. It focusses on the gastrin-releasing peptide receptor found in multiple cancers, including but not limited to prostate, breast, and ovarian types. The GRPr is present in up to 100% of prostate cancers, even in cases where PSMA expression is absent.
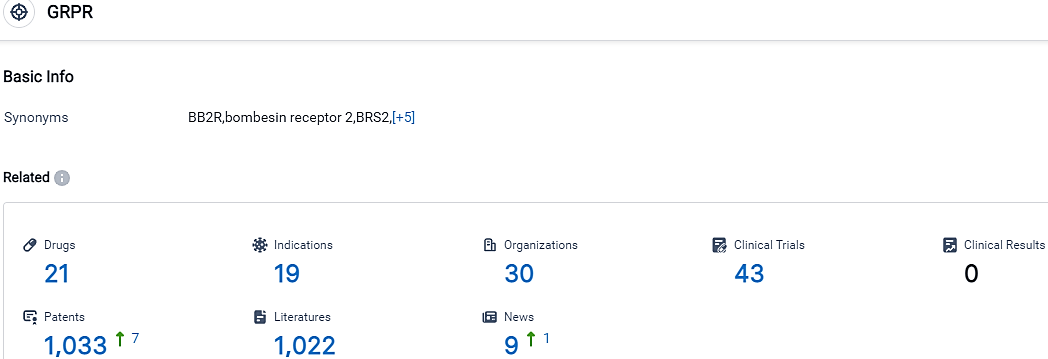
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of October 14, 2023, there are 21 investigational drugs for the GRPR target, including 19 indications, 30 R&D institutions involved, with related clinical trials reaching 43,and as many as 1033 patents.
SAR-Bombesin shows potential as a therapeutic radiopharmaceutical drug targeting the GRPR. Its active indications in prostatic cancer, contrast agents, and metastatic breast cancer highlight its potential in treating these specific conditions. However, further research and clinical trials are needed to fully assess the drug's safety and efficacy.