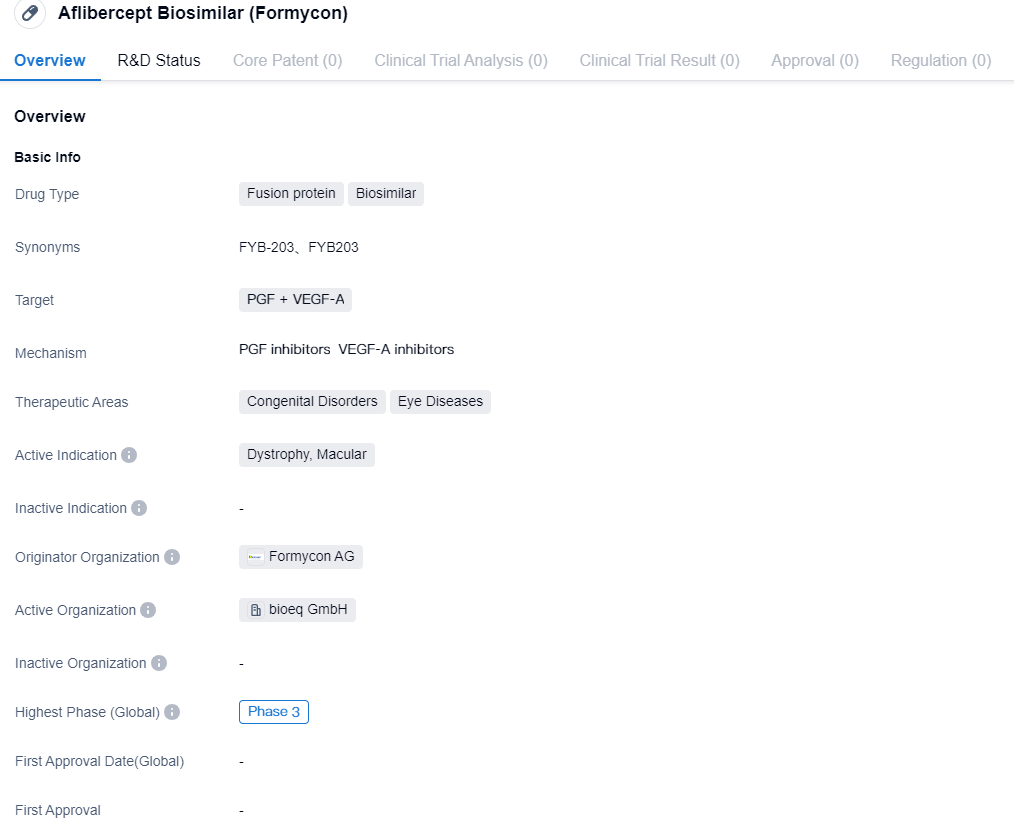
Formycon announces File Acceptance for FYB203 by the U.S. FDA
Formycon AG, along with its licence ally Klinge Biopharma GmbH (“Klinge”), has revealed that the FDA has given a green light to the biologics license application (“BLA”) for FYB203, a biosimilar to Eylea® (Active Ingredient: aflibercept). The file acceptance stage has been reached, with the intended action date set for June 2024.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Eylea® is employed to treat neovascular age-related macular degeneration and other critical retina conditions. It inhibits the action of vascular endothelial growth factor, causing a reduction of excessive blood vessels formation in the retina. Eylea®, with a global sales record of about US$ 9.6 billion, stands as the leading drug within this treatment scope.
The FDA's file acceptance signifies a significant step in pursuing the approval of our second biosimilar, FYB203, in the field of ophthalmology in the US. FYB203 promises to enrich the breadth of our ophthalmic collection and caters to an essential and growing market for severe retinal disease treatment, notes Formycon's CEO, Dr Stefan Glombitza.
Formycon is a premium independent producer of superior biopharmaceutical drugs, especially biosimilars. The firm prioritises treatments for ophthalmology, immunology, and other notable chronic ailments, overseeing the process from technical development through to clinical phase III and marketing approval dossier preparation. Via its biosimilars, Formycon is committed to ensuring wide access to vital and cost-effective medicines for as many patients as possible. Currently, Formycon is influencing the development of six biosimilars. Leveraging on its vast experience in biopharmaceutical drug development, they are also contributing to COVID-19 drug development, FYB207.
Biosimilars refer to the follow-on versions of biopharmaceuticals after patent lapse. Since the 1980s, innovation in biopharmaceuticals has radically improved the treatment of life-threatening diseases like cancer, diabetes, rheumatoid arthritis, multiple sclerosis and many eye diseases. By 2025, drugs which generate approximately USD 100 billion in revenue will lose their patent protection. Regulatory pathways in heavily regulated markets (such as the EU, US, Japan, Canada, Australia) approve of these bio-products based on their proven similarity to the original biopharmaceutical reference product. Currently, biosimilars generate a global sales of over $15 billion. Forecasts project that by 2030, this could rise to over $74 billion.
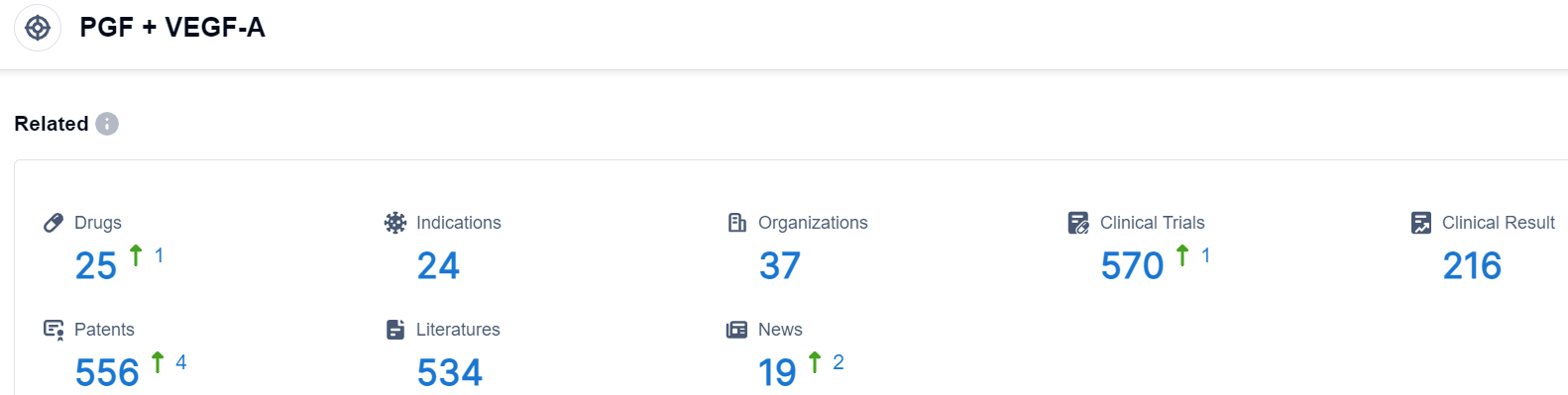
Click on the image below for direct access to the latest R&D progress on PGF+VEGF-A target drugs, indications, research institutions, clinical trials, and more. as of August 30, 2023, there are a total of 25 drugs under research for the PGF+VEGF-A target, covering 24 indications, 37 research institutions involved, involves 216 related clinical trials, and up to 556 patents. Risankizumab (AbbVie/Boehringer Ingelheim) is a humanized anti-interleukin (IL)-23 subunit p19 monoclonal antibody that binds and neutralizes the p19 subunit of IL-23. It is being developed in psoriasis and Crohn’s disease.