Formycon initiates Phase III clinical trial for Keytruda® biosimilar candidate FYB206
Formycon AG disclosed that the initial participant has been registered in the Phase III clinical investigation "Lotus" to assess the safety and effectiveness of FYB206/pembrolizumab versus the popular immuno-oncology medication Keytruda. The double-blind, multi-center "Lotus" trial is assessing the optimal overall response rate in individuals diagnosed with non-small cell lung cancer.
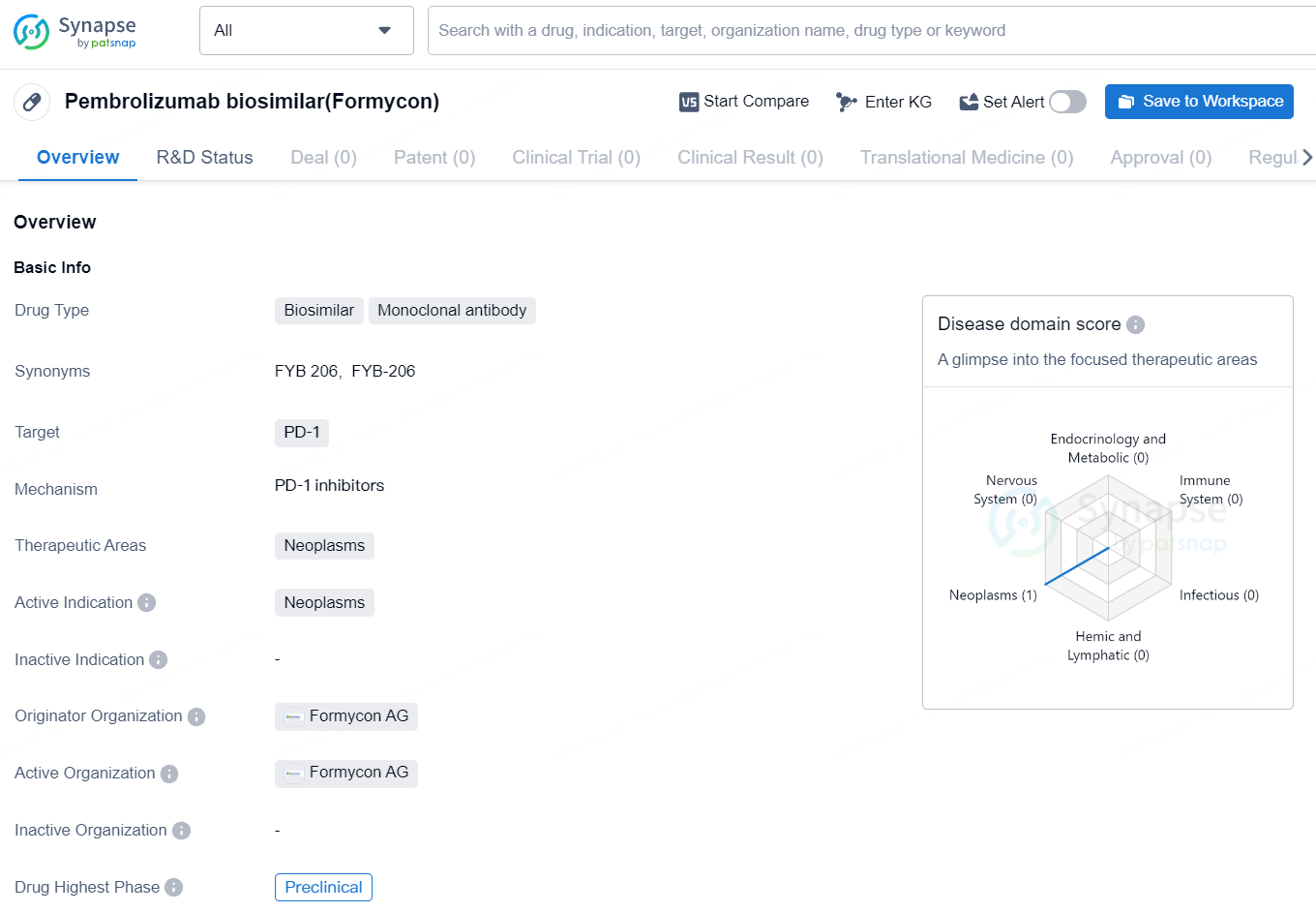
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The comparative regimen involves a maximum of 17 treatment cycles over a period of 40 weeks. Changes in tumor size will be continuously monitored using imaging techniques throughout this time frame. Subsequent to the comparative treatment, therapy will be extended for an additional 12 months.
The study protocol was formulated in close collaboration with the regulatory bodies, including the U.S. Food and Drug Administration, European Medicines Agency, and Japanese Pharmaceuticals and Medical Devices Agency. The trial is underway in multiple Eastern European and Southeast Asian nations, running concurrently with a Phase I study that commenced in mid-June, comparing the pharmacokinetics, safety, and tolerability of FYB206 with the reference drug Keytruda in patients with malignant melanoma.
As Dr. Andreas Seidl, Chief Scientific Officer of Formycon AG, notes, the launch of the Phase III clinical trial marks a significant achievement in the pursuit of delivering an efficient, safe, and cost-effective treatment option for numerous severely ill cancer patients globally in the coming years.
Non-small cell lung cancer is just one of the many cancer types for which the immune checkpoint inhibitor pembrolizumab is prescribed. A multitude of cancers thwart the body's immune responses by manipulating immune checkpoints, against which pembrolizumab, targeting the PD-1 immune checkpoint, reinstates the ability of the body's T cells to combat and eliminate the cancer cells.
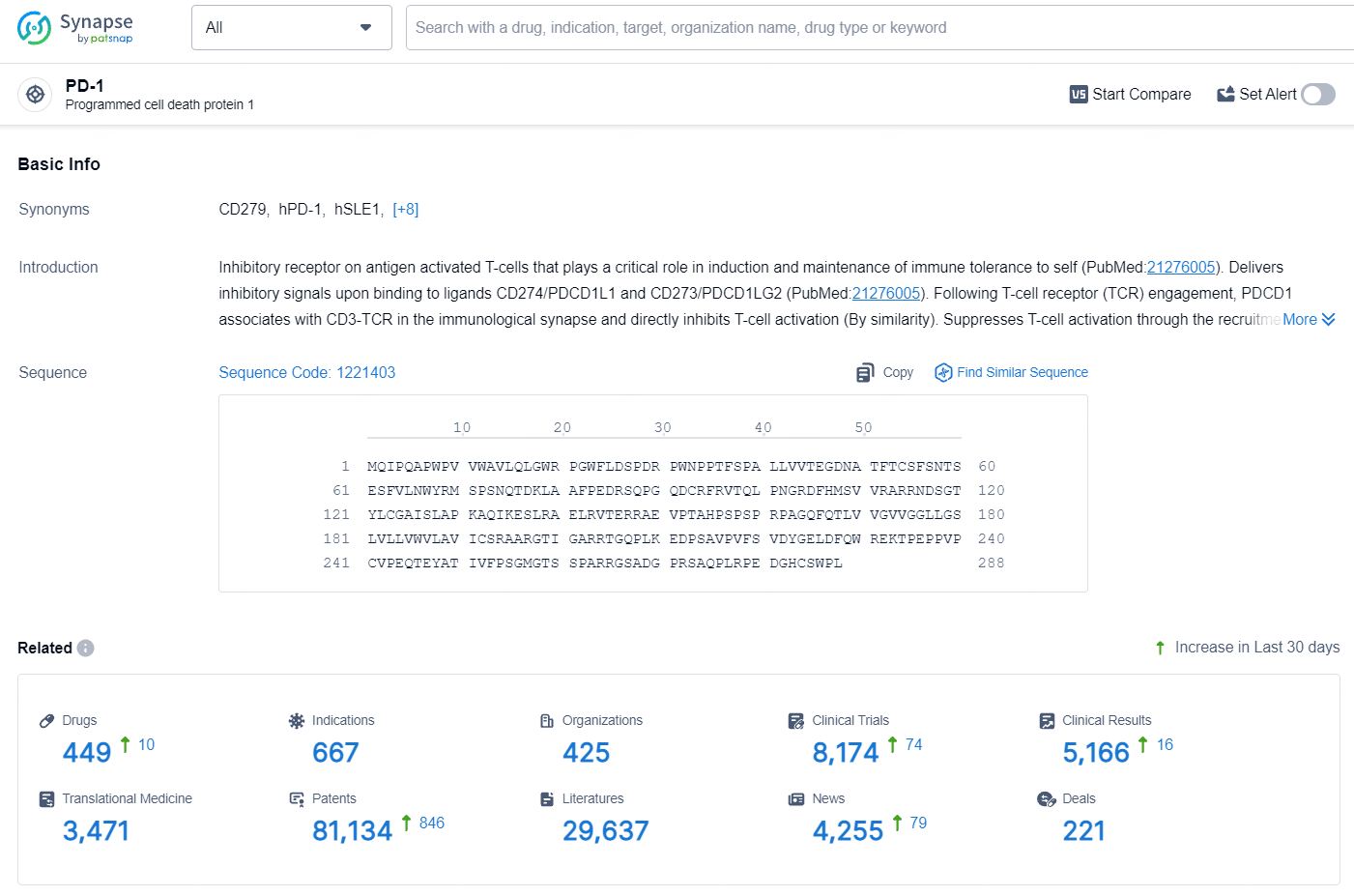
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of August 2, 2024, there are 449 investigational drugs for the PD-1 target, including 667 indications, 425 R&D institutions involved, with related clinical trials reaching 8174, and as many as 81134 patents.
FYB206 represents an emerging drug in the field of biomedicine, specifically in the treatment of cancer. As it progresses through the preclinical phase, further clinical trials and development activities will be needed to assess its safety and efficacy in treating neoplasms. The drug has the potential to offer alternative therapeutic options for patients with various types of cancers, pending successful development and regulatory approval.