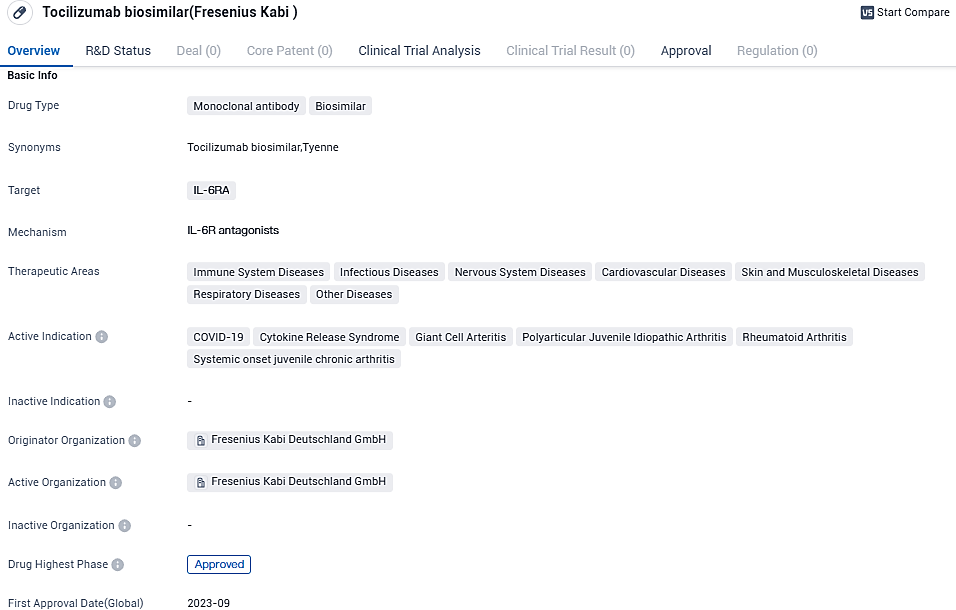
Fresenius Kabi introduces Tyenne*, the inaugural EU-sanctioned tocilizumab biosimilar
Fresenius Kabi, a worldwide healthcare firm with a focus on biopharmaceuticals, clinical nutrition, medical technologies, and I.V. generic drugs for severe and long-term illnesses, made an announcement about the introduction of Tyenne®, its tocilizumab biosimilar which references the RoActemra® (tocilizumab) in the European Union.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Tyenne® is recognized as Europe's first accessible tocilizumab biosimilar, offering treatment for a variety of inflammatory and immune conditions, including rheumatoid arthritis, giant cell arteritis, polyarticular juvenile idiopathic arthritis, systemic juvenile idiopathic arthritis, and cytokine release syndrome, as well as providing assistance in treating COVID-19.
Tyenne® is the third biosimilar from Fresenius Kabi that gained approval and hit the European market. It presents a versatile suite of easy-to-use subcutaneous and intravenous delivery methods to patients and healthcare professionals.
Fresenius Kabi's CEO, Pierluigi Antonelli, commented, “The roll-out validates our Vision 2026 expansion tactic to supply indispensable treatment alternatives to worldwide patients and healthcare providers. Our biopharma portfolio continually expands, made evident by the introduction of tocilizumab on the European scene, underscoring our steady growth trajectory. Our standing as the inaugural healthcare company to present a tocilizumab biosimilar in the EU displays our determination to lead the biopharma sector.”
Dr. Michael Schönhofen, President of Fresenius Kabi Biopharmaceuticals, added, “We take great pride in being the pioneers in furnishing an economic, top-quality, secure, and alternate tocilizumab therapy solution for patients suffering from inflammatory and immune disorders and healthcare professionals.
Tyenne®, offering both subcutaneous and intravenous routes, stands to revolutionize the treatment scene, augment patient results, and alleviate the financial stress on healthcare systems and patients."
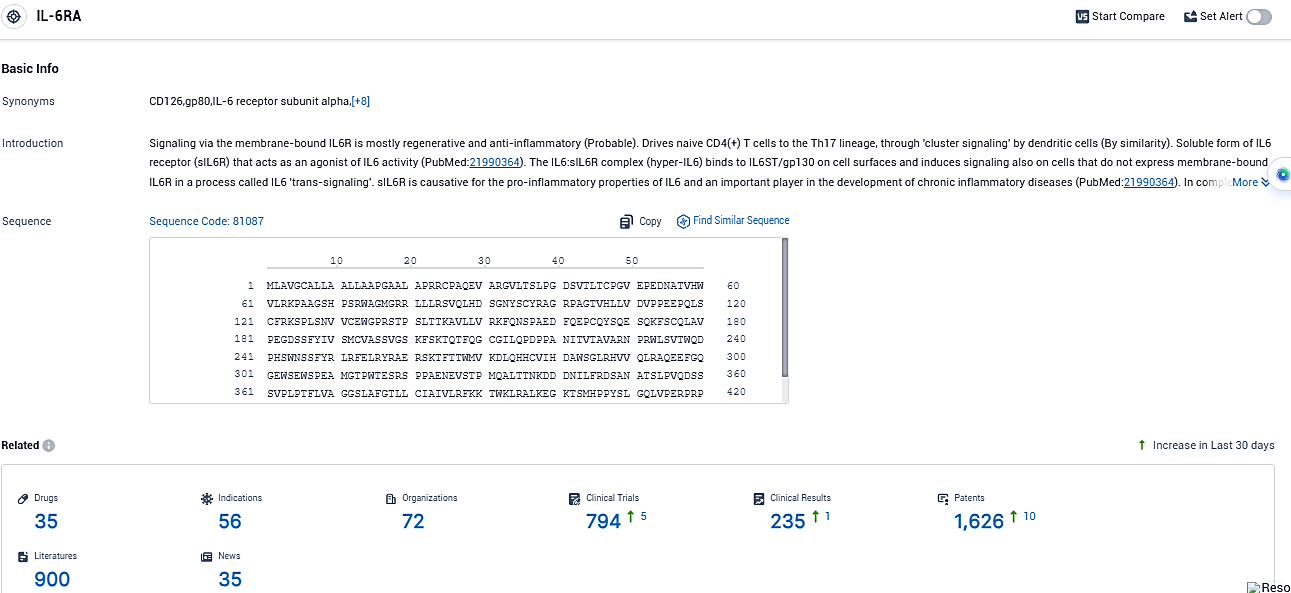
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 9, 2023, there are 35 investigational drugs for the IL-6RA target, including 56 indications, 72 R&D institutions involved, with related clinical trials reaching 794, and as many as 1626 patents.
Tyenne® (tocilizumab), is a biosimilar to the reference medicinal product RoActemra®, a prescription medicine called an Interleukin-6 (IL-6) receptor antagonist. Tocilizumab is a biological therapy approved in the EU for use in the treatment of various inflammatory and immune mediated conditions, including rheumatoid arthritis, giant cell arteritis, polyarticular juvenile idiopathic arthritis.