Gan & Lee's GZR18 Injection Achieves 17.29% Weight Loss in Phase IIb Trial
Gan & Lee Pharmaceuticals recently declared that their independently created long-acting GLP-1 receptor agonist (GLP-1 RA), GZR18 injection, showed favorable outcomes in a clinical study involving adults with obesity/overweight in China.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The phase IIb clinical trial was a multi-center, randomized, double-blind, placebo-controlled study conducted across 25 clinical trial sites in China, involving a total of 340 participants. The subjects included overweight individuals with at least one weight-related comorbidity or obese adults with poorly controlled diet and exercise regimens.
Participants were randomized to receive bi-weekly doses of 12 mg, 18 mg, 24 mg, 48 mg, or a once-weekly dose of 24 mg GZR18 injection, or a placebo for 30 weeks. The primary efficacy outcome measured was the percentage change in body weight from baseline after 30 weeks of treatment. Additional assessments included changes in average weight, waist circumference, waist-to-hip ratio, body mass index, glycemic parameters, and the safety and tolerability of the drug.
After 30 weeks of treatment, all groups receiving different doses and frequencies of GZR18 injection demonstrated a significant reduction in the percentage change in body weight from baseline compared to the placebo group. The mean percentage changes in body weight from baseline were -11.15%, -13.22%, -14.25%, -17.29%, and -17.78%, each showing greater effectiveness than the placebo group. There was no significant difference observed in the mean percentage change in body weight between the group receiving 48 mg bi-weekly and the group receiving 24 mg weekly.
The bi-weekly GZR18 injections were generally well-tolerated and safe, in line with known safety profiles of GLP-1 receptor agonists. The most frequently reported adverse events were gastrointestinal reactions, which were mostly mild to moderate in severity, comparable to other GLP-1 RAs.
The full results of this phase IIb study are expected to be released later this year and will be published in a peer-reviewed journal.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
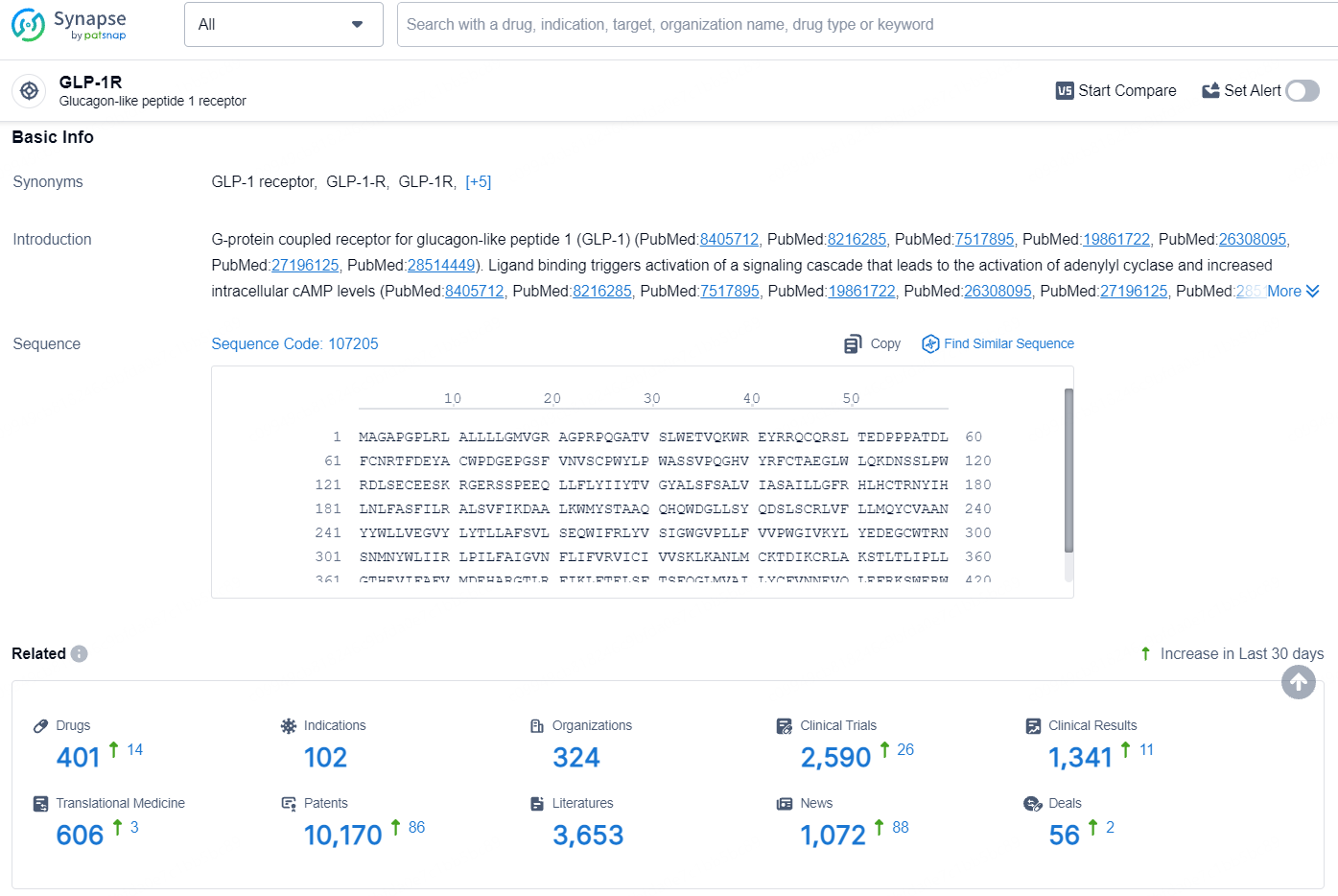
According to the data provided by the Synapse Database, As of July 24, 2024, there are 401 investigational drugs for the GLP-1 RA target, including 102 indications, 324 R&D institutions involved, with related clinical trials reaching 2590, and as many as 10170 patents.
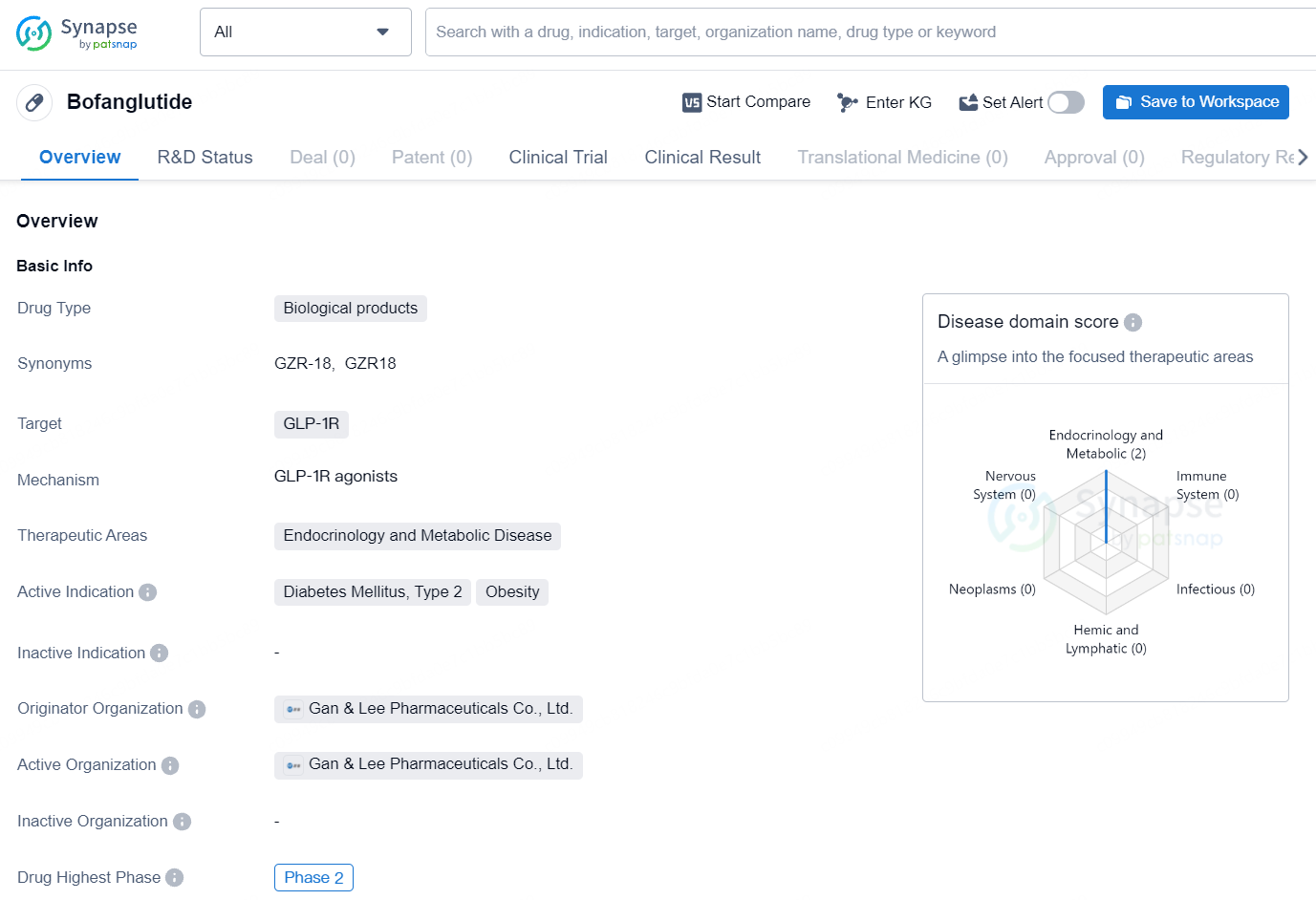
Bofanglutide is a biological product drug that targets GLP-1R, making it suitable for treating conditions related to endocrinology and metabolic disease. Bofanglutide presents a snapshot of its current development status and therapeutic focus. In the continuously evolving field of biomedicine, staying informed about the progress and potential of drugs like Bofanglutide is essential for making strategic business decisions in the pharmaceutical industry.