Grit Biotech's GT201 Receives FDA and Chinese IND Approval for Genetically Modified TIL Therapy
Grit Biotechnology, a prominent clinical-stage company specializing in tumor-infiltrating lymphocyte (TIL) therapies, has reached a significant achievement with its genetically modified TIL product, GT201. After obtaining approval for its investigational new drug (IND) application in China in July 2023, GT201 has also secured IND clearance from the U.S. FDA, enabling the commencement of clinical trials in the United States.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
GT201, a cutting-edge genetically engineered TIL product, has been developed through Grit Biotechnology's proprietary platforms StemTexp® and StaViral®. This product enhances T cell longevity and functionality by incorporating a crucial membrane-bound cytokine complex. Compared to conventional TIL therapies, GT201 demonstrates superior proliferation, tumor-killing capability, and long-term survival, with less reliance on IL-2. Recently, the U.S. FDA and China Center for Drug Evaluation (CDE) have approved the GT201 IND for clinical trials in advanced solid tumor patients in both countries.
Grit Biotechnology, a clinical-stage cell therapy company headquartered in China, is at the forefront of TIL therapy innovation. The company's development efforts for TIL and other cell therapies are underpinned by core technological platforms including StemTexp® (a proprietary TIL expansion platform that maintains stemness), StaViral® (a stable virus transduction system), KOReTIL® (an efficient CRISPR knockout system), and ImmuT Finder® (a genome-wide CRISPR/Cas screening platform). These platforms facilitate the creation of next-generation gene-edited TIL products and a variety of cell therapies.
Moreover, Grit’s non-genetically engineered TIL program, GT101, is currently undergoing Phase 2 (pivotal) trials and is projected to submit a Biologics License Application (BLA) by 2025, positioning it as the leading TIL pipeline in China.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
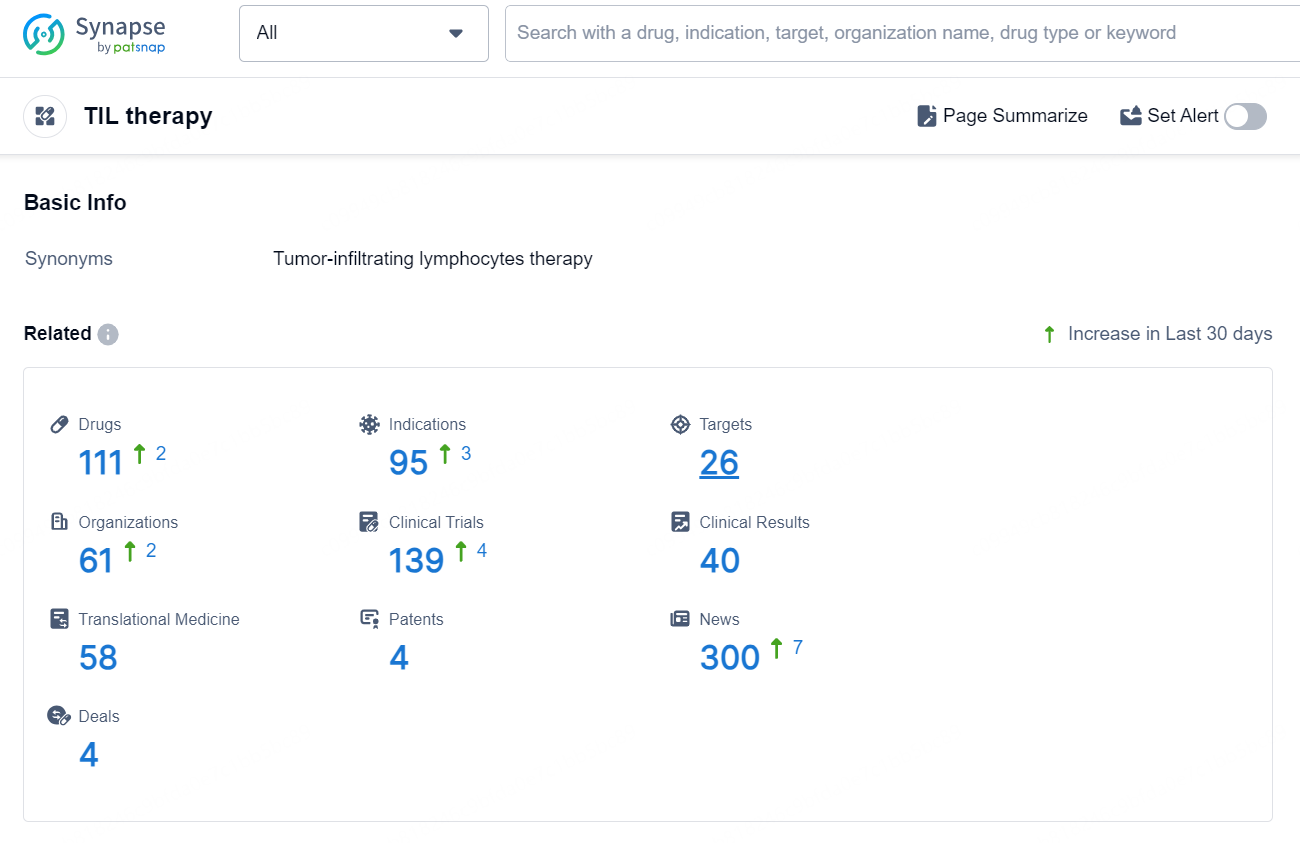
According to the data provided by the Synapse Database, As of September 3, 2024, there are 111 investigational drugs for the TIL therapy, including 95 indications, 26 targets, 61 R&D institutions involved, with related clinical trials reaching 139, and as many as 4 patents.
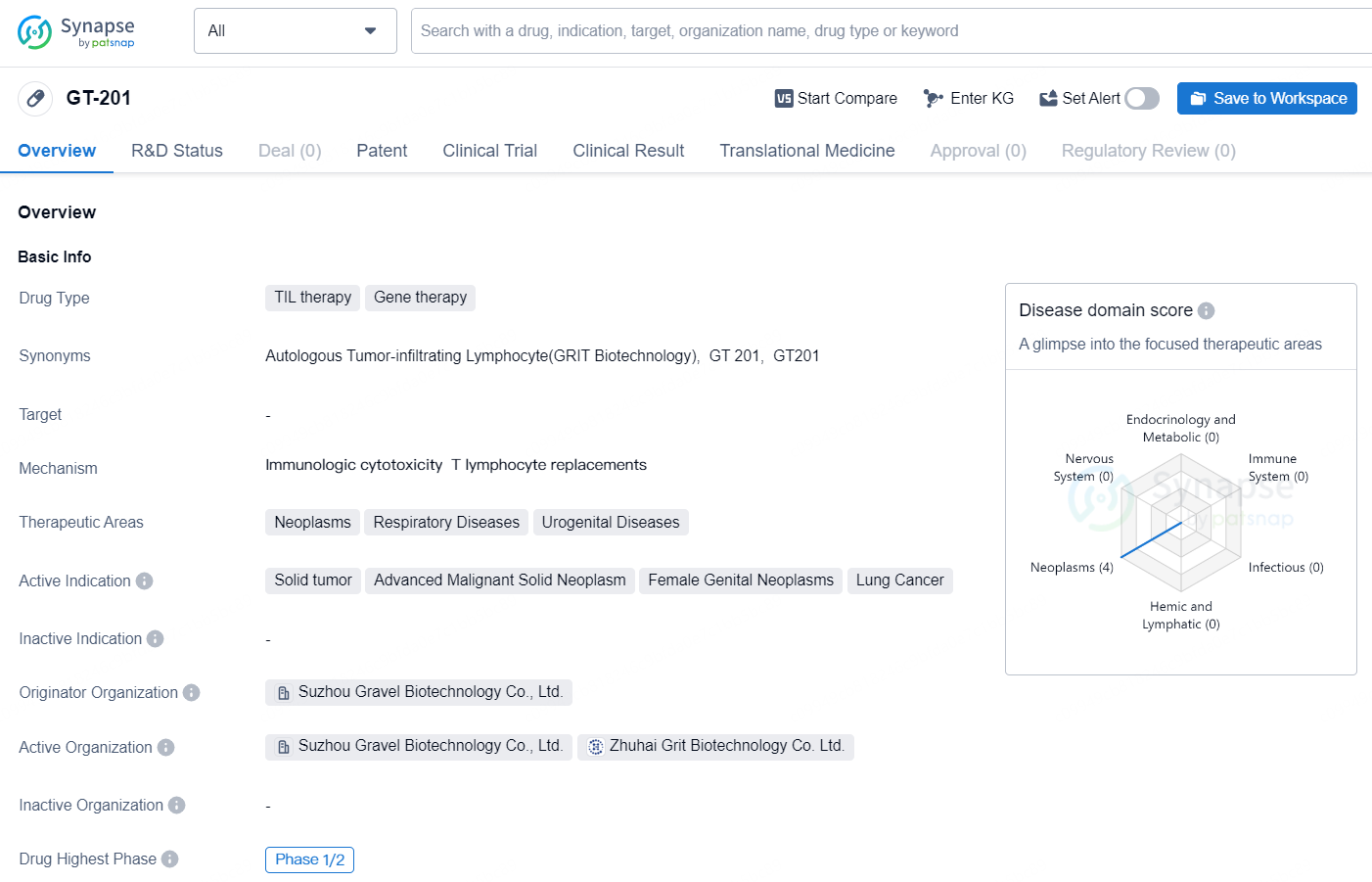
GT-201 is a TIL (tumor infiltrating lymphocytes) therapy and gene therapy drug being developed by Suzhou Gravel Biotechnology Co., Ltd. It is being investigated for the treatment of various neoplastic and respiratory diseases, as well as urogenital diseases. The drug is currently in the Phase 1/2 of clinical development globally, with the same phase in progress in China as well. The active indications for GT-201 include solid tumors, advanced malignant solid neoplasms, female genital neoplasms, and lung cancer.