GSK has secured an exclusive licensing deal with Hansoh Pharmaceutical for the compound HS-20093
GSK plc has formed an exclusive partnership with Hansoh Pharma, which is renowned for its dedication to creating transformative therapeutic solutions aimed at aiding individuals battling severe medical conditions. Under the terms of this collaboration, both entities will focus on the further development and commercialization of a novel therapeutic agent, HS-20093, which functions as an antibody-drug conjugate (ADC) specifically targeting B7-H3, and is coupled with a topoisomerase inhibitor-based payload that has previously shown clinical efficacy.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Hesham Abdullah, a top executive in charge of Global Oncology Research and Development at GSK, stated: "A considerable number of solid cancers exhibit a high level of B7-H3 expression, highlighting the urgent need for innovative therapies. Our team is keen to further the development of this promising therapeutic candidate for various cancer types and to potentially integrate it with our existing product range in future strategic combined treatments.”
Through a recent deal, GSK has secured the exclusive global license to advance the clinical trials and market HS-20093. This acquisition equips GSK with an additional ADC in clinical phases, augmenting their portfolio and enhancing their ability to develop treatments that fulfill the yet unaddressed needs in solid cancer care.
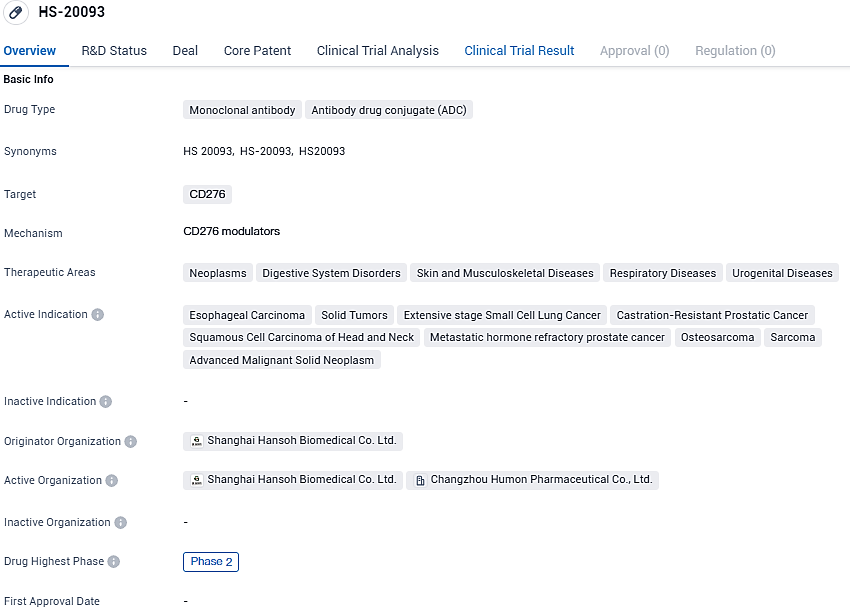
As of now, HS-20093 is under evaluation in phase I and II studies conducted in China. At the 2023 American Society of Clinical Oncology's yearly meeting, findings from the ARTEMIS-001 phase I study targeting advanced solid cancers with HS-20093 were unveiled, showing promising clinical outcomes particularly in small-cell lung carcinoma, non-small-cell lung carcinoma, and sarcoma. These findings included multiple validated responses and a tolerable safety profile.
Eliza Sun, a high-ranking official on the Hansoh Pharma Board, expressed enthusiasm regarding the fresh licensing contract with GSK, saying: “The innovative HS-20093, directed against B7-H3, has demonstrated hopeful preliminary outcomes in the treatment of lung cancer. Our collaboration with GSK, with whom we have an ongoing licensing deal for HS-20089, supports Hansoh’s dedication to offering a groundbreaking treatment alternative to cancer sufferers worldwide.”
In a development from October 2023, GSK and Hansoh Pharma agreed to collaborate on HS-20089, a B7-H4 ADC in phase II clinical trials. HS-20089 shows potential to lead its class, particularly in ovarian and endometrial cancer treatments, with the prospect of applying it to additional solid cancer types.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
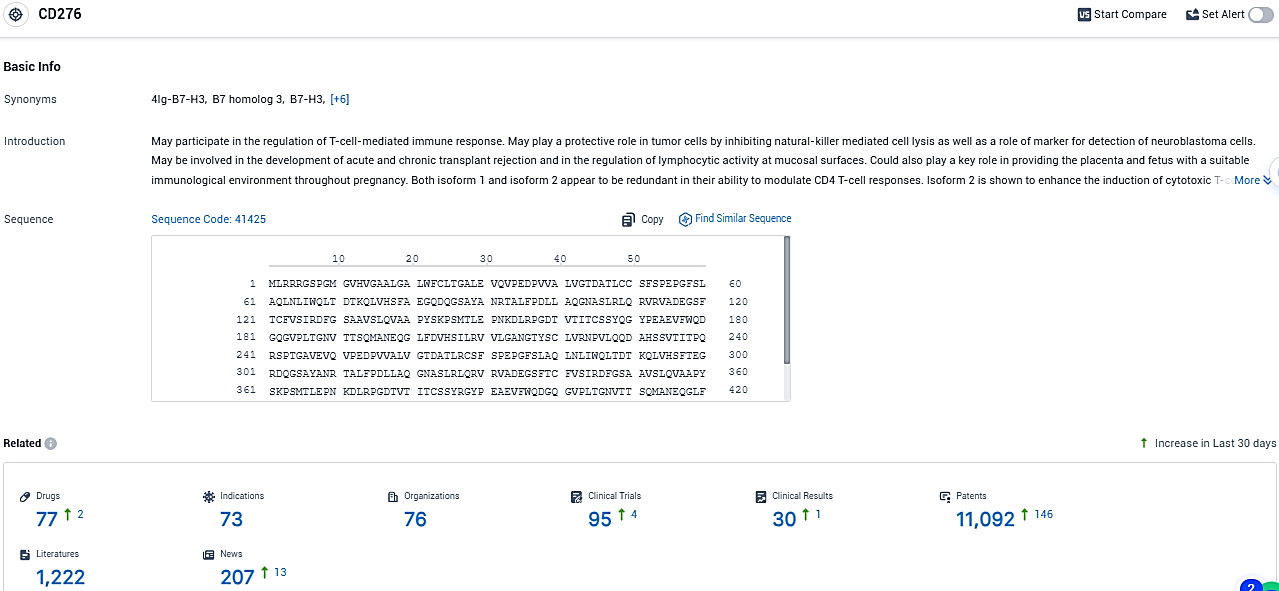
According to the data provided by the Synapse Database, As of December 29, 2023, there are 77 investigational drugs for the CD276 target, including 73 indications, 76 R&D institutions involved, with related clinical trials reaching 95, and as many as 11092 patents.
HS-20093 is a novel B7-H3-targeted antibody-drug conjugate composed of a fully humanised anti-B7-H3 monoclonal antibody covalently linked to topoisomerase inhibitor payload. HS-20093 is being developed for the treatment of lung cancer, sarcoma, head and neck cancers and other solid tumours in multiple phase I and II clinical trials in China.