Health Canada Approves Entos Pharmaceuticals to Start COVID-19 Vaccine Booster Trial
Entos Pharmaceuticals, a biotechnology firm in the clinical stage that focuses on genetic therapies using its unique Fusogenix PLV nucleic acid delivery system, along with its collaborator Aegis Life, Inc., have announced that Entos has secured authorization from Health Canada to commence a phase 1/2 clinical study assessing Covigenix VAX-002, an experimental COVID-19 booster shot.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Formulated using the Entos Fusogenix PLV delivery system, Covigenix VAX-002 is a plasmid DNA-based vaccine designed for optimal expression of key SARS-CoV-2 antigens, with particular emphasis on the currently prevalent omicron variants. Aegis holds global rights for the vaccine outside of Canada and will partner with Entos for its clinical advancement.
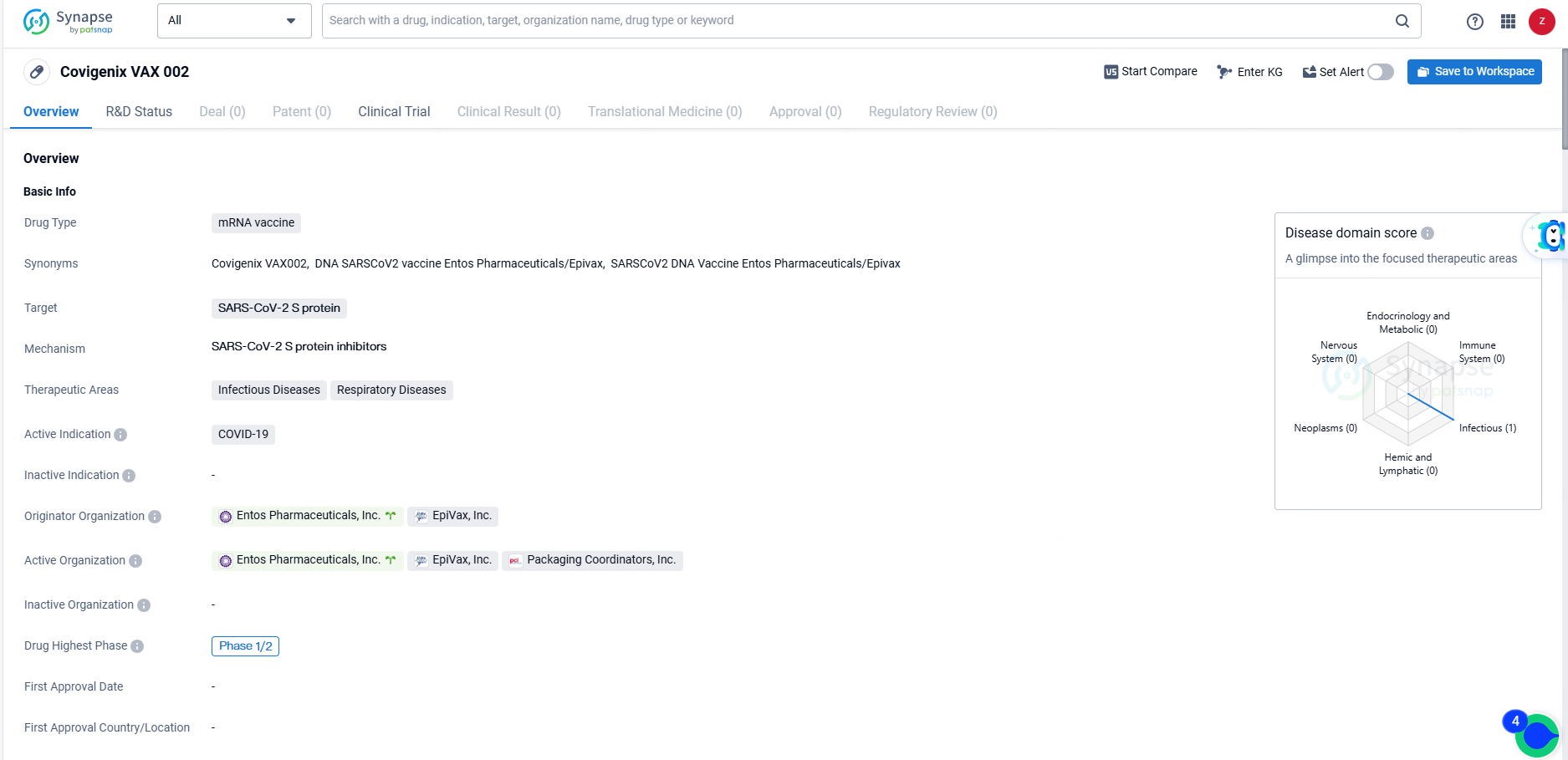
The clinical study for the Covigenix VAX-002 COVID-19 booster follows a phase 1/2 structure. The phase 1 segment aims to enroll 50 participants to determine the optimal booster dose of Covigenix VAX-002. In the phase 2 phase, 250 participants are expected to be enrolled to assess the safety and immunogenic response of the optimal dose identified in phase 1. Findings from this study will guide future intramuscular dosing of Covigenix formulations and support future global development strategies.
"COVID-19 continues to be a significant public health challenge," stated Steve Chen, M.D., Chief Medical Officer at Entos. "Elderly and immunocompromised individuals need a COVID-19 vaccine that offers long-lasting robust protection. Developing countries equally require a fridge-stable vaccine that can be quickly deployed. Covigenix VAX-002 has the potential to be the premier DNA fridge-stable vaccine providing prolonged protection compared to any other vaccine currently available."
The booster vaccine is being manufactured at the Company's Good Manufacturing Practices (GMP) facility in Carlsbad, California. This facility was established by Entos to support the clinical development of its genetic medicine initiatives, including Covigenix VAX-002, clinical candidates for collaborative programs, as well as future candidates in rare diseases, ocular and ophthalmic conditions, oncology, and infectious diseases.
"The Health Canada approval for advancing Covigenix VAX-002 into clinical trials is a significant milestone for Entos," said Arun Raturi, Ph.D., Chief Scientific Officer at Entos. "This will be the first GMP clinical vaccine produced entirely in-house at Entos. The phase 1/2 study will not only yield valuable insights into the utility of Covigenix VAX-002 for patients needing a COVID-19 booster but also serve to further validate the Fusogenix platform as a foundation for future vaccine and therapeutic development."
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
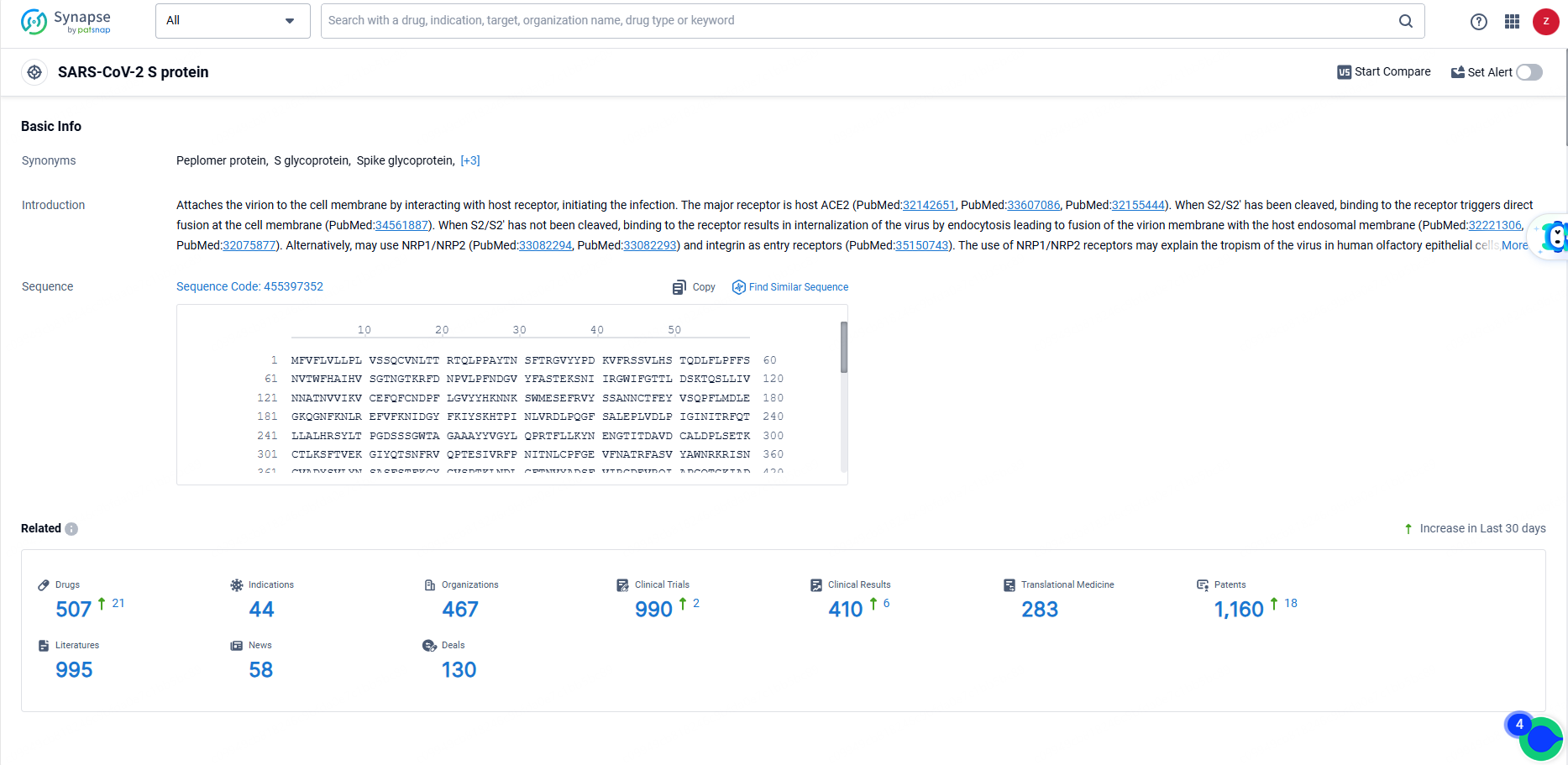
According to the data provided by the Synapse Database, As of June 14, 2024, there are 507 investigational drugs for the SARS-CoV-2 S protein targets, including 44 indications, 467 R&D institutions involved, with related clinical trials reaching 990, and as many as 1160 patents.
Covigenix VAX 002 is an mRNA vaccine targeting the SARS-CoV-2 S protein, with a primary indication for COVID-19. The drug has reached Phase 1/2 in its global development, reflecting its progress in clinical testing. As a potential solution to the ongoing COVID-19 pandemic, Covigenix VAX 002 holds promise for addressing critical unmet medical needs and presents opportunities for strategic partnerships and commercialization.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!