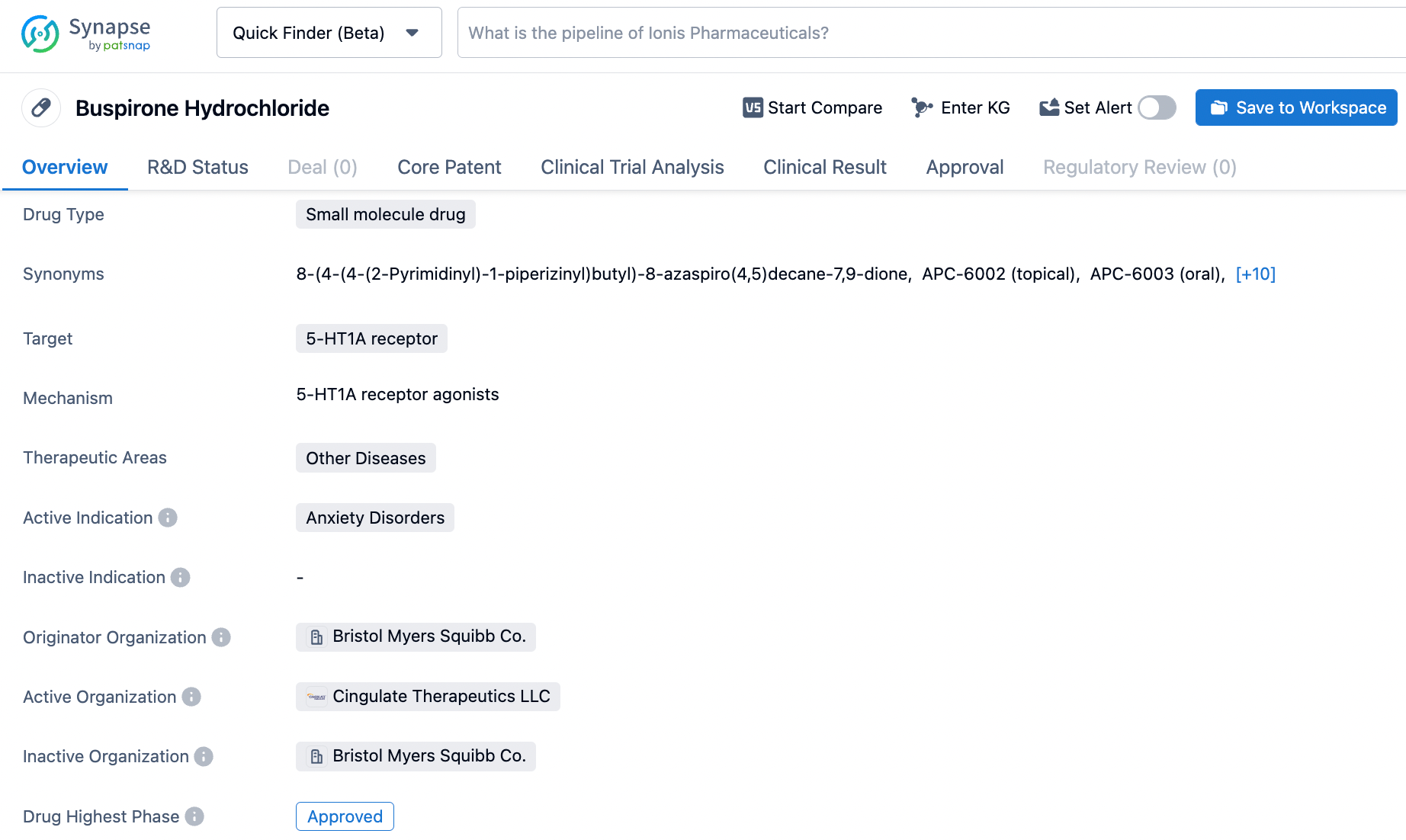
How to Effectively Search for BuSpar on Synapse
BuSpar® (buspirone hydrochloride) is an antianxiety agent that is not chemically or pharmacologically related to the benzodiazepines, barbiturates, or other sedative/anxiolytic drugs. Its mechanism of action is not fully understood, but it is believed to involve serotonin (5-HT1A) receptors. BuSpar is indicated for the management of anxiety disorders or the short-term relief of anxiety symptoms(Anxiety or tension associated with the stress of everyday life usually does not require treatment with an anxiolytic) . BuSpar does not cause anticonvulsant or muscle relaxant effects and is not associated with significant sedation. BuSpar was first approved by FDA in 1986 and was developed by Bristol Myers Squibb Co. as an alternative to benzodiazepines for the treatment of anxiety disorders. Click on the image below to begin the exploration journey of BuSpar through the Synapse database!
You can search for the latest pharmaceutical information such as drugs, targets, patents, transactions, clinical results, etc. through the Synapse database. Come and experience it!